Abstract
Germline mutations in BRCA1 and BRCA2 (BRCA1/2) genes are present in about 50% of cases of hereditary breast cancer. Proteins encoded by these genes are key players in DNA repair by homologous recombination (HR). Advances in next generation sequencing and gene panels for breast cancer testing have generated a large amount of data on gene variants implicated in hereditary breast cancer, particularly in genes such as PALB2, ATM, CHEK2, RAD51, MSH2, and BARD1. These genes are involved in DNA repair. Most of these variants have been reported for Caucasian, Jewish, and Asian population, with few reports for other communities, like those in Latin American (LA) countries. We reviewed 81 studies from 11 LA countries published between 2000 and 2019 but most of these studies focused on BRCA1/2 genes. In addition to these genes, breast cancer-related variants have been reported for PALB2, ATM, CHEK2, BARD1, MLH1, BRIP1, MSH2, NBN, MSH6, and PMS2 genes. Some of these variants are unique to LA populations. This analysis may contribute to enhance breast cancer variant characterization, and thus to find therapies and implement precision medicine for LA communities.
Keywords: breast cancer, BRCA1, BRCA2, DNA repair, Latin America, germline, PARP inhibitors therapy
1. Introduction
Breast cancer is the leading cause of cancer death in women worldwide, with about 627,000 deaths in 2018 [1]. About 5–10% of all breast cancer is caused by germline variants in BRCA1/2 [2,3]. Moreover, about 50% of hereditary breast cancer (HBC) cases present germline mutations in BRCA genes [2]. BRCA1/2 pathogenic variants confer more than 50% risk of breast cancer development [4]. Since the identification of breast cancer genes BRCA1/2 in 1994 and 1995, respectively [5,6,7], a large amount of data on the risks conferred by these genes for breast and other cancers has been generated. These genes play an important role in the repair of double-strand breaks in DNA by homologous recombination (HR) along with a plethora of additional proteins [2,8,9,10].
About 50–60% of cases of HBC show variants in BRCA1/2 genes. The remaining percentage involves moderate and low penetrance variants in non-BRCA genes [11]. Next generation sequencing (NGS) and other technologies are still identifying variants in genes associated with breast cancer. Non-BRCA variants have been observed in genes like ATM, PALB2, RAD51, and BARD1 [4,12,13]. Like BRCA genes, these genes codify for proteins participating in DNA repair [14,15].
The National Comprehensive Cancer Network (NCCN) guidelines for genetic assessment in hereditary breast cancer (version 3.2019) recommends genetic evaluation of ATM, CDH1, CHEK2, NBN, NF1, and PALB2 in addition to BRCA1/2 genes. There is accumulative evidence that variants in BARD1, BRIP1, MSH2, MLH1, MSH6, PMS2, RAD51C, and RAD51D are also causative of HBC, although some of these gene products participate in other DNA repair pathways and there is insufficient evidence for establishing management strategies [16]. This generates a paradigm for genetic counseling and assessment. Based on the absence of functional studies evaluating the activity of these genes, genetic advisory is being challenged by patient management.
Notably, most of the data on HBC variants represent Caucasian populations [17]. HBC accounts for about 15% of breast cancer cases in Latin America (LA) [18]. This population, resulting from the combination of Native American, Spanish, African, and other communities, is highly heterogeneous, even within the regions of the different countries where they live [19,20,21]. This diversity impacts the genetic variation involved in HBC in this large community [20]. Unfortunately, the data on HBC variants for this population is scarce and mainly focused on BRCA1/2; few cumulative reports on these variants have been reported. This information will be required to implement programs of precision medicine for HBC patients belonging to this population, like those that are being implemented for prevention and therapy of BRCA1/2 variants, particularly with Poly-ADP ribose polymerase (PARP) inhibitors [22,23,24,25,26,27]. Additionally, studies are suggesting that some tumor types with mutations in DNA repair genes are amenable for immunotherapy approaches [28,29,30,31,32,33]. This review analyzes 81 studies of germline mutations in breast cancer from 11 LA countries published between 2000 and 2019, with the focus on BRCA and non-BRCA genes involved in DNA repair.
2. Materials and Methods
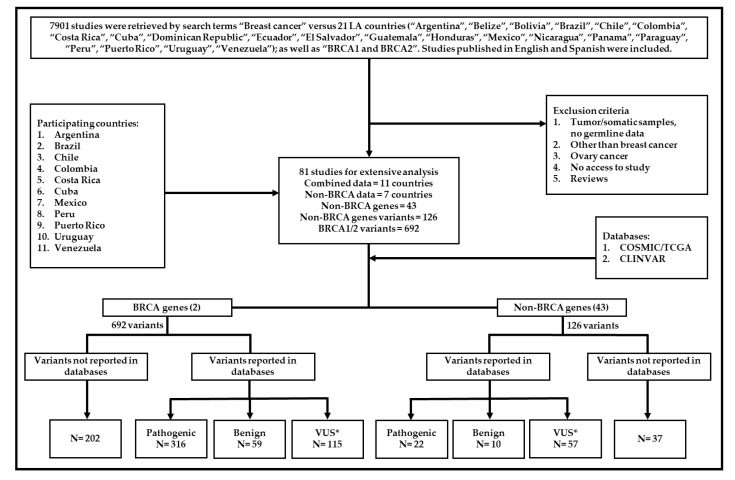
We searched the PubMed database (https://www.ncbi.nlm.nih.gov/pubmed/) for all breast cancer studies in 21 LA countries. Studies published between 2000 and April 5, 2019 were considered. A total of 7901 studies were retrieved using the following search terms “Breast cancer” and 21 LA countries (“Argentina”, “Belize”, “Bolivia”, “Brazil”, “Chile”, “Colombia”, “Costa Rica”, “Cuba”, “Dominican Republic”, “Ecuador”, “El Salvador”, “Guatemala”, “Honduras”, “Mexico”, “Nicaragua”, “Panama”, “Paraguay”, “Peru”, “Puerto Rico”, “Uruguay”, and “Venezuela”); as well as “BRCA1 and BRCA2”. Studies published in English and Spanish were included. Titles and abstracts were reviewed, and ineligible reports were discarded. The inclusion criteria considered research papers, case reports, and germline mutation data. A diagram showing the process of data acquisition is presented in Figure 1.
Figure 1.
Study diagram. This diagram shows data acquisition and variant classification. *VUS: conflicting interpretation, uncertain significance, likely pathogenic, likely benign, risk factor, drug response.
Considering the inclusion criteria, 81 studies were selected for extensive analysis [34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114]. Eleven countries reported germline gene variant data in breast cancer (Argentina, Brazil, Chile, Colombia, Costa Rica, Cuba, Mexico, Peru, Puerto Rico, Uruguay, and Venezuela). Six hundred and ninety-two variants were found in BRCA1/2 genes. Additionally, 126 variants were reported in 43 non-BRCA genes of patients from 7 countries. All variants were investigated in the Catalogue of Somatic Mutations in Cancer (COSMIC) (https://cancer.sanger.ac.uk/cosmic) and the ClinVar (https://www.ncbi.nlm.nih.gov/clinvar/) databases. The COSMIC database is the largest curated resource, with cancer mutation data from over 32,000 genomes including samples from The Cancer Genome Atlas (TCGA) and the International Cancer Genome Consortium (ICGC). ClinVar was used to measure the clinical significance of the reported data.
Among variants in BRCA1/2 genes, 202 were not found in the databases, 316 were classified as pathogenic, 59 as benign, and 115 were classified as “conflicting interpretations”, “uncertain significance”, “likely pathogenic”, and “likely benign”. Regarding the variants in non-BRCA genes, 22 variants were classified as pathogenic, 10 as benign, 57 as “conflicting interpretations”, “uncertain significance”, “likely pathogenic”, “likely benign”, “drug response”, “risk” and “risk factor”, and 37 were not found in the databases. Variants classified as “conflicting interpretations”, “uncertain significance”, “likely pathogenic”, “likely benign”, “drug response”, “risk”, and “risk factor”, were grouped as variants of unknown significance (VUS) for the purposes of this study.
3. Results
3.1. The Scope of Germline Mutations in Breast Cancer in LA Countries
From our literature analysis, only 11 out of 21 countries documented germline data for breast cancer cases. Brazil led the list of reports (32), followed by Chile, Mexico, Colombia, and Argentina. Cuba and Venezuela only had one report each (Table 1). No germline data were found for studies performed in Ecuador, Paraguay, Panama, Dominican Republic, El Salvador, Bolivia, Guatemala, Nicaragua, Honduras, and Belize. Most of the data for germline variants in HBC accounts for the BRCA1/2 genes and just a few studies include non-BRCA genes in a few LA countries.
Table 1.
BRCA and Non-BRCA genes papers in Latin America (LA).
| Country | Total Retrieved Papers 1 | Germline Data 2 | BRCA1/2 Papers | Non-BRCA Papers | Total Papers | References | |
|---|---|---|---|---|---|---|---|
| 1 | Brazil | 3290 | ✔ | 13 | 19 | 32 | [34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65] |
| 2 | Chile | 455 | ✔ | 7 | 7 | 14 | [66,67,68,69,70,71,72,73,74,75,76,77,78,79] |
| 3 | Mexico | 2014 | ✔ | 8 | 4 | 12 | [80,81,82,83,84,85,86,87,88,89,90,91] |
| 4 | Colombia | 274 | ✔ | 5 | 1 | 6 | [92,93,94,95,96,97] |
| 5 | Argentina | 893 | ✔ | 4 | 4 | [98,99,100,101] | |
| 6 | Peru | 161 | ✔ | 2 | 1 | 3 | [102,103,104] |
| 7 | Puerto Rico | 253 | ✔ | 2 | 1 | 3 | [105,106,107] |
| 8 | Uruguay | 126 | ✔ | 3 | 3 | [108,109,110] | |
| 9 | Costa Rica | 56 | ✔ | 2 | 2 | [111,112] | |
| 10 | Cuba | 142 | ✔ | 1 | 1 | [113] | |
| 11 | Venezuela | 76 | ✔ | 1 | 1 | [114] | |
| 12 | Ecuador | 42 | |||||
| 13 | Paraguay | 31 | |||||
| 14 | Panama | 23 | |||||
| 15 | Dominican Republic | 18 | |||||
| 16 | El Salvador | 16 | |||||
| 17 | Bolivia | 13 | |||||
| 18 | Guatemala | 8 | |||||
| 19 | Nicaragua | 7 | |||||
| 20 | Honduras | 2 | |||||
| 21 | Belize | 1 | |||||
| Total | 7901 | 11 | 48 | 33 | 81 |
1 First search combining terms “Breast cancer” versus 21 LA countries (“Argentina”, “Belize”, “Bolivia”, “Brazil”, “Chile”, “Colombia”, “Costa Rica”, “Cuba”, “Dominican Republic”, “Ecuador”, “El Salvador”, “Guatemala”, “Honduras”, “Mexico”, “Nicaragua”, “Panama”, “Paraguay”, “Peru”, “Puerto Rico”, “Uruguay”, “Venezuela”); as well as “BRCA1 and BRCA2”. 2 Second search including papers with germline mutation data and breast cancer.
3.2. Genes reported for HBC in LA countries
The study found 363 variants in BRCA2 and 329 variants in BRCA1 in 11 countries (Argentina, Brazil, Chile, Colombia, Costa Rica, Cuba, Mexico, Peru, Puerto Rico, Uruguay, and Venezuela). In addition, variants were also observed in 43 non-BRCA genes like ATM, TP53, CHEK2, BARD1, MLH1, PALB2, BRIP1, MSH2, MSH6, NBN, and PMS2 in Chile, Brazil, Colombia, and Mexico (Table 2, Figure S1).
Table 2.
Breast Cancer gene variants reported by LA countries in breast cancer.
| Gene | Number of Variants | Argentina | Brazil | Chile | Colombia | Costa Rica | Cuba | Mexico | Peru | Puerto Rico | Uruguay | Venezuela |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BRCA2 | 363 | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ |
| BRCA1 | 329 | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ |
| ATM | 17 | ✔ | ✔ | ✔ | ✔ | |||||||
| TP53 | 11 | ✔ | ✔ | ✔ | ||||||||
| CHEK2 | 8 | ✔ | ✔ | ✔ | ✔ | |||||||
| BARD1 | 6 | ✔ | ✔ | ✔ | ✔ | ✔ | ||||||
| MLH1 | 6 | ✔ | ✔ | ✔ | ||||||||
| PALB2 | 6 | ✔ | ✔ | |||||||||
| BRIP1 | 5 | ✔ | ✔ | |||||||||
| MSH2 | 4 | ✔ | ✔ | |||||||||
| MSH6 | 4 | ✔ | ✔ | |||||||||
| NBN | 4 | ✔ | ✔ | |||||||||
| PMS2 | 4 | ✔ | ||||||||||
| APC | 3 | ✔ | ||||||||||
| ATR | 3 | ✔ | ✔ | |||||||||
| BLM | 3 | ✔ | ||||||||||
| RAD51C | 3 | ✔ | ✔ | ✔ | ||||||||
| CDKN2A | 2 | ✔ | ||||||||||
| ERCC1 | 2 | ✔ | ✔ | |||||||||
| ERCC2 | 2 | ✔ | ✔ | ✔ | ||||||||
| ERCC3 | 2 | ✔ | ||||||||||
| FANCB | 2 | ✔ | ||||||||||
| FANCI | 2 | ✔ | ||||||||||
| LIG4 | 2 | ✔ | ||||||||||
| MUTYH | 2 | ✔ | ||||||||||
| RAD51B | 2 | ✔ | ✔ | |||||||||
| RAD51D | 2 | ✔ | ✔ | |||||||||
| XRCC1 | 2 | ✔ | ✔ | |||||||||
| CDH1 | 1 | ✔ | ||||||||||
| FANCC | 1 | ✔ | ||||||||||
| FANCF | 1 | ✔ | ||||||||||
| FANCL | 1 | ✔ | ||||||||||
| FANCM | 1 | ✔ | ||||||||||
| MSH3 | 1 | ✔ | ||||||||||
| NF1 | 1 | ✔ | ||||||||||
| POLH | 1 | ✔ | ||||||||||
| POLQ | 1 | ✔ | ||||||||||
| PTEN | 1 | ✔ | ||||||||||
| RAD50 | 1 | ✔ | ||||||||||
| RAD51 | 1 | ✔ | ✔ | |||||||||
| RECQL4 | 1 | ✔ | ||||||||||
| SMAD4 | 1 | ✔ | ||||||||||
| WRN | 1 | ✔ | ||||||||||
| XPC | 1 | ✔ | ||||||||||
| XRCC3 | 1 | ✔ | ✔ |
3.3. BRCA1 and BRCA2 Genes in LA countries
Studies conducted in some LA countries have established frequent and founder BRCA1/2 mutations in the region. The BRCA1 3450del4, c.5123C>A variant and BRCA2 3034del4 are considered founder mutations in Colombia [67,92,115]. The variants 5382insC in BRCA1 and 6633del5 and 156_157insAlu in BRCA2 are prevalent in Brazil [52,105,115,116]. Three variants observed in the Ashkenazi community, c.66_67delAG and c.5263insC in BRCA1, and c.5946delT in BRCA2 were reported in Argentina [92,98,105]. In Mexico, the BRCA1 ex9–12 deletion is reported as a founder mutation [88], while the variants, 2805_2808delAGAT and 3124_3133delAGCAATATTA in BRCA1, and 2639_2640delTG and 5114_5117delTAAA in BRCA2 are reported as pathogenic [89,105,115]. In Puerto Rico, the variant E1308X in BRCA2 is present in most cases of HBC [105], while in Chile, the variants c.3331_3334delCAAG and c.3759dupT in BRCA1 and c.4740_4742dupTG, c.5146_5149delTATG in BRCA2 are more prevalent [67]. In Peru, three recurrent mutations were described, 185delAG and 2080delA in BRCA1, and mutation 3034del4 in BRCA2 have been observed [102,116]. In Costa Rica, BRCA2 variants such as the 5531delTT are frequently reported [111,116]. No recurrent mutations in BRCA1/2 genes were found in Venezuela [114,116]. No reports on BRCA1/2 variants were registered for Belize, Bolivia, the Dominican Republic, Ecuador, El Salvador, Guatemala, Honduras, Nicaragua, Panama, and Paraguay. This evidence suggests that some variants are preferentially distributed in particular LA countries.
Some other BRCA1/2 variants are observed in more than one LA country (Table 3), like the pathogenic variant BRCA2 c.2808_2811del variant reported in seven countries (Argentina, Brazil, Colombia, Mexico, Peru, Uruguay, and Venezuela) and the pathogenic BRCA1 variants c.68_69delAG and c.211A>G observed in six countries. Remarkably, the BRCA2 variant c.7469T>C classified as benign in ClinVar was observed in HBC cases in Argentina, Brazil, Colombia, Cuba, Mexico, Uruguay, and Venezuela, prompting a reconsideration of its reclassification. In summary, there are shared and specific BRCA1/2 variants in HBC patients, reflecting the ethnic heterogeneity in Latin America [105].
Table 3.
Frequent BRCA1/2 gene variants in LA.
| Gene | rs | Exon | Argentina | Brazil | Chile | Colombia | Costa Rica | Cuba | Mexico | Peru | Puerto Rico | Uruguay | Venezuela |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BRCA1 VARIANT | |||||||||||||
| c.68_69delAG | rs386833395 | 2 | (4) | (4) | (2) | (1) | (1) | (1) | |||||
| c.181T>G | rs28897672 | 5 | (1) | (4) | (1) | ||||||||
| c.211A>G | rs80357382 | 5 | (2) | (3) | (1) | (3) | (1) | (1) | |||||
| c.3113A>G | rs16941 | 11 | (1) | (3) | (2) | (1) | |||||||
| c.3548A>G | rs16942 | 11 | (1) | (3) | (1) | (1) | |||||||
| c.1067A>G | rs1799950 | 11 | 2 | (3) | (1) | ||||||||
| c.3119G>A | rs4986852 | 11 | (1) | (3) | (1) | (1) | (1) | ||||||
| c.2612C>T | rs799917 | 11 | (1) | (3) | (1) | ||||||||
| c.3331_3334delCAAG | rs80357701 | 11 | (9) | (3) | (6) | ||||||||
| c.4308C>T | rs1060915 | 13 | (1) | (2) | (1) | ||||||||
| c.4837A>G | rs1799966 | 16 | (1) | (3) | (1) | (1) | |||||||
| c.5123C>A | rs28897696 | 18 | (1) | (2) | (8) | (3) | |||||||
| c.5266dupC | rs397507247 | 20 | (4) | (16) | (1) | (1) | |||||||
| BRCA2 VARIANT | |||||||||||||
| c.865A>C | rs766173 | 10 | (1) | (2) | (1) | (1) | |||||||
| c.2971A>G | rs1799944 | 11 | (1) | (2) | (2) | (1) | (1) | ||||||
| c.5744C>T | rs4987117 | 11 | (1) | (2) | (2) | (1) | (1) | ||||||
| c.2808_2811del | rs80359351 | 11 | (3) | (5) | (2) | (1) | (2) | (1) | (1) | ||||
| c.5351dupA | rs80359507 | 11 | (1) | (2) | (2) | ||||||||
| c.5946delT | rs80359550 | 11 | (5) | (4) | (1) | (4) | (1) | ||||||
| c.7469T>C | rs11571707 | 15 | (2) | (4) | (1) | (1) | (1) | (1) | (1) | ||||
| c.10234A>G | rs1801426 | 27 | (1) | (3) | (2) | (1) |
() Number of reporting papers.
There are also reports of large genomic rearrangements (LGR) in BRCA1/2 in LA countries (Table 4). Brazil has reported 18 different LGR in BRCA genes, more than in other LA countries. The deletion of exons 1-2 in BRCA1 was reported in three cases in Brazil and Puerto Rico [56,63,106]. The 6kb duplication in exon 13 in BRCA1 was reported in seven Brazilian patients [51]. In Mexico, the exon 9–12 deletion in BRCA1 is considered as a founder mutation that has been found in 25 cases so far [80,81,84,88]. Interestingly, the LGR g.26826_30318del in BRCA2 found in Brazil was associated with high-risk male breast cancer [35,64]. There are also descriptions of LGRs in BRCA1/2 in breast cancer patients from Peru, Puerto Rico, and Uruguay [104,106,109].
Table 4.
Large genomic rearrangements in BRCA1/2.
| Gene | Mutation | Cases | Brazil | Colombia | Mexico | Peru | Puerto Rico | Uruguay | References |
|---|---|---|---|---|---|---|---|---|---|
| BRCA1 | exon 1-2 deletion | 3 | ✔ | ✔ | [60,63,106] | ||||
| BRCA1 | exon 3 deletion | 2 | ✔ | [60,63] | |||||
| BRCA1 | exon 4-6 deletion | 2 | ✔ | [60,63] | |||||
| BRCA1 | exon 5-7 deletion | 2 | ✔ | [53,60] | |||||
| BRCA1 | exon 8 deletion | 2 | ✔ | [60,63] | |||||
| BRCA1 | exon 8-13 deletion | 1 | ✔ | [104] | |||||
| BRCA1 | exon 9-11 deletion | 1 | ✔ | [80] | |||||
| BRCA1 | exon 9-12 deletion | 25 | ✔ | [80,81,84,88] | |||||
| BRCA1 | exon 9-19 deletion | 2 | ✔ | [41,60] | |||||
| BRCA1 | exon 12 deletion | 1 | ✔ | [80] | |||||
| BRCA1 | exon 14 deletion | 1 | ✔ | [109] | |||||
| BRCA1 | exon 14-16 deletion | 1 | ✔ | [60] | |||||
| BRCA1 | exon 16-17 deletion | 2 | ✔ | [60,65] | |||||
| BRCA1 | exon 18-19 deletion | 2 | ✔ | ✔ | [60,104] | ||||
| BRCA1 | exon 19 deletion | 1 | ✔ | [60] | |||||
| BRCA1 | exon 21-23 deletion | 1 | ✔ | [60] | |||||
| BRCA1 | exon 24 duplication | 1 | ✔ | [65] | |||||
| BRCA1 | 6-KB DUP EX13 | 7 | ✔ | [51] | |||||
| BRCA2 | exon 1 deletion | 3 | ✔ | [80] | |||||
| BRCA2 | exon 1-14 deletion | 2 | ✔ | [94] | |||||
| BRCA2 | exon 2 deletion | 1 | ✔ | [60] | |||||
| BRCA2 | exon 11 deletion | 1 | ✔ | [80] | |||||
| BRCA2 | exon 13 deletion | 1 | ✔ | [60] | |||||
| BRCA2 | exon 14 deletion | 1 | ✔ | [60] | |||||
| BRCA2 | exon 15-16 deletion | 1 | ✔ | [92] | |||||
| BRCA2 | exon 17 deletion | 1 | ✔ | [80] | |||||
| BRCA2 | exon 22-24 deletion | 2 | ✔ | [80] | |||||
| BRCA2 | exon 23 deletion | 2 | ✔ | [80] | |||||
| BRCA2 | exon 25 deletion | 1 | ✔ | [60] | |||||
| BRCA2 | exon 26 deletion | 1 | ✔ | [80] | |||||
| BRCA2 | g.26826_30318del | 2 | ✔ | [35,64] |
3.4. Non-BRCA Genes Reported in Breast Cancer in LA Countries
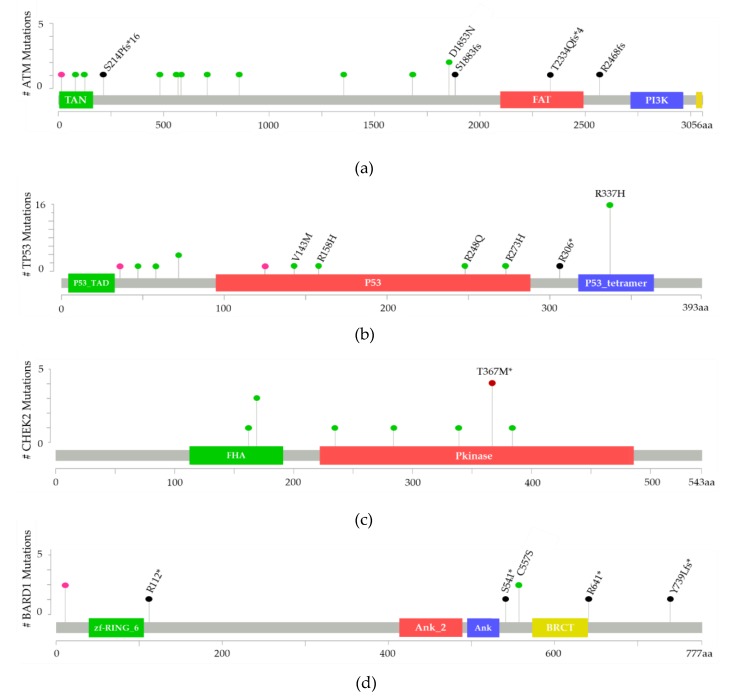
Brazil, Chile, Colombia, Mexico, Peru, Puerto Rico, and Uruguay are the only LA countries that have conducted screenings for non-BRCA genes in breast cancer cases. We found 126 variants in 43 non-BRCA genes (Table 5). Variants were found in ATM, TP53, CHEK2, BARD1, MLH1, PALB2, and BRIP1. The most frequent variants of ATM, TP53, CHEK2, and BARD1 are represented in Figure 2 as lollipop plots following published instructions [117,118]. The ataxia telangiectasia mutated (ATM) gene was the most reported in LA breast cancer cases. Seventeen variants were found in ATM in Mexico, Brazil, Chile, and Colombia, 11 variants in TP53 (Brazil, Colombia, and Uruguay) and 8 variants in CHEK2 (Brazil, Chile, Mexico, and Uruguay). Furthermore, six variants in each of the following genes were found in HBC cases: BARD1, MLH1, PALB2 and five variants in BRIP1 (Brazil, Chile, Colombia, Mexico, Peru, and Uruguay). The pathogenic classification of these variants is described according to ClinVar and COSMIC databases in Table S1. The CHEK2 variant c.1100delC reported in Brazil and Chile presents conflicting interpretations of pathogenicity according to ClinVar and is not reported in the COSMIC database. The TP53 variant c.1010G>A (p.R337H) observed in Brazil is considered to be pathogenic by ClinVar, but it is not classified as such by COSMIC [115].
Table 5.
Frequent variants in non-BRCA genes in LA.
| Gene | rs | Variant | COSMIC | CLINVAR | Brazil | Chile | Colombia | Mexico | Peru | Uruguay |
|---|---|---|---|---|---|---|---|---|---|---|
| ATM | NA | c.634delT | ✔ | x | ||||||
| ATM | NA | c.5648_5655del | x | |||||||
| ATM | rs145119475 | c.4060C>A | ✔ | x | ||||||
| ATM | rs1800056 | c.2572T>C | ✔ | ✔ | x | |||||
| ATM | rs1801516 | c.5557G>A | ✔ | ✔ | x | x | ||||
| ATM | rs1801673 | c.5558A>T | ✔ | ✔ | x | |||||
| ATM | rs200381392 | c.1703G>T | ✔ | x | ||||||
| ATM | rs202173660 | c.1444A>C | ✔ | ✔ | x | |||||
| ATM | rs2234997 | c.378T>A | ✔ | ✔ | x | |||||
| ATM | rs2235006 | c.1744T>C | ✔ | x | ||||||
| ATM | rs4986761 | c.2119T>C | ✔ | ✔ | x | |||||
| ATM | rs587782153 | c.5039C>T | ✔ | ✔ | x | |||||
| ATM | rs758962678 | c.241A>G | ✔ | x | ||||||
| ATM | rs759965045 | c.7702_7703del | ✔ | x | ||||||
| ATM | rs771887195 | c.43del | ✔ | x | ||||||
| ATM | rs786203421 | c.7000_7003delTACA | ✔ | x | ||||||
| ATM | rs786204433 | c.5644C>T | ✔ | ✔ | x | |||||
| TP53 | rs1042522 | c.215C>G | ✔ | ✔ | x | |||||
| TP53 | rs11540652 | c.743G>A | ✔ | ✔ | x | |||||
| TP53 | rs121912664 | c.1010G>A | ✔ | ✔ | x | |||||
| TP53 | rs121913344 | c.916C>T | ✔ | ✔ | x | |||||
| TP53 | rs144386518 | c.173C>G | ✔ | ✔ | x | |||||
| TP53 | rs1800370 | c.108G>A | ✔ | ✔ | x | |||||
| TP53 | rs1800371 | c.139C>T | ✔ | ✔ | x | |||||
| TP53 | rs28934576 | c.818G>A | ✔ | ✔ | x | |||||
| TP53 | rs55863639 | c.375G>A | ✔ | ✔ | x | |||||
| TP53 | rs587782144 | c.473G>A | ✔ | ✔ | x | |||||
| TP53 | rs587782620 | c.427G>A | ✔ | ✔ | x | |||||
| CHEK2 | NA | c.1015C>T | x | |||||||
| CHEK2 | NA | c.1151delT | x | |||||||
| CHEK2 | NA | c.705A>C | x | |||||||
| CHEK2 | NA | c.852G>T | x | |||||||
| CHEK2 | rs1555926890? | c.506T>C | x | |||||||
| CHEK2 | rs555607708 | c.1100delC | ✔ | x | x | |||||
| CHEK2 | rs587781652 | c.485A>G | ✔ | x | ||||||
| CHEK2 | rs864622149 | c.846+1G>C | ✔ | x | ||||||
| BARD1 | NA | c.2215dupT | x | |||||||
| BARD1 | rs143914387 | c.33G>T | x | |||||||
| BARD1 | rs28997576 | c.1670G>C | x | |||||||
| BARD1 | rs587781948 | c.1921C>T | x | |||||||
| BARD1 | rs758972589 | c.334C>T | ✔ | ✔ | x | |||||
| BARD1 | rs777937955 | c.1622C>A | x | |||||||
| MLH1 | NA | c.1966C>T | x | |||||||
| MLH1 | NA | c.413A>G | x | |||||||
| MLH1 | NA | c.791G>A | x | |||||||
| MLH1 | rs148317871 | c.2213G>A | ✔ | x | ||||||
| MLH1 | rs63751615 | c.676C>T | ✔ | x | ||||||
| MLH1 | del_exon8 | NA | NA | x | ||||||
| PALB2 | NA | c.1861C>A | x | |||||||
| PALB2 | NA | c.483C>G | x | |||||||
| PALB2 | rs150390726 | c.23C>T | ✔ | x | ||||||
| PALB2 | rs152451 | c.1676A>G | ✔ | x | ||||||
| PALB2 | rs180177100 | c.1240C>T | ✔ | ✔ | x | |||||
| PALB2 | rs45551636 | c.2993C>T | x | |||||||
| BRIP1 | rs202072866 | c.415T>G | ✔ | ✔ | x | |||||
| BRIP1 | rs28997569 | c.790C>T | ✔ | x | ||||||
| BRIP1 | rs371185409 | c.3079G>A | ✔ | x | ||||||
| BRIP1 | rs45589637 | c.2220G>T | ✔ | x | ||||||
| BRIP1 | rs759031349 | c.689C>T | ✔ | x |
✔Databases; x Country; NA: Not available.
Figure 2.
Mutations in non-BRCA genes reported in LA breast cancer patients. Frequently reported mutations are observed in ATM, TP53, CHEK2 and BARD1 genes. (a) ATM likely loss of function and therefore likely oncogenic mutations S214Pfs*16, S1883fs, T2334Qfs*4, and R2568fs; (b) TP53 likely loss of function and therefore likely oncogenic mutations V143M, R158H, R248Q, R273H, R306*, and R337H; (c) CHEK2 most reported mutation T367M*; (d) BARD1 likely loss of function and therefore likely oncogenic mutations R112*, S541*, RG41* and Y739Lfs*. Mutation type: missense (green), truncating (black), in frame (red), other (pink).
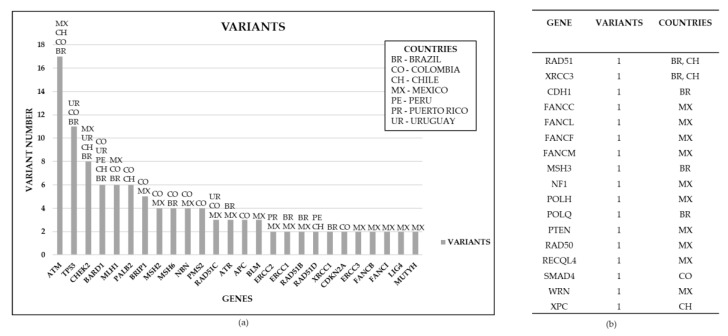
Non-BRCA genes with at least two reported variants in LA countries are presented in Figure 3A. Mexico, Brazil, and Colombia are the countries with more reported variants in non-BRCA genes. Non-BRCA genes with only one variant reported in LA are described in Figure 3B. A total of 10 non-BRCA genes with one variant are reported in Mexico [81]. Additionally, only one variant was found in RAD51, XRCC3, CDH1, MSH3, POLQ, SMAD4, and XPC genes in Brazil, Chile, and Colombia.
Figure 3.
Non-BRCA gene variants reported from breast cancer patients in 7 LA countries. (a) Reported number of germline genes variants in breast cancer by country in LA; (b) Unique gene variants reported in LA countries.
Cock et al. [92] reported germline mutations in non-BRCA genes (PALB2, ATM, MSH2, PMS2, MSH6, and TP53) in Colombia. Among their findings, the mutation c.137G>T in PMS2 was identified in a patient with early-onset breast cancer (31 years old), an invasive ductal carcinoma, HR-, HER2+, and positive family history. Another patient diagnosed at 36 years with invasive ductal carcinoma HR+, HER2-, had a PALB2 mutation c.1240C>T. There was another patient diagnosed at 48 years with invasive ductal carcinoma and positive history of familial cancer carrying the ATM c.43del variant. VUS were also reported in patients carrying PMS2, BARD1, RAD51C, BRIP1, MSH6, and MSH2 variants [92]. A previously study of Mexican patients carried out by Quezada et al. describes variants in DNA repair genes including ATM, ERCC3, FANCI, ATR, MLH1, NBN, RAD51C, non-BRCA genes (54%) and BRCA1/2 (46%) [81].
4. Biallelic Cases, Double Heterozygosity and Young Women in LA Countries
In addition to cancer-associated variants in BRCA1/2 and non-BRCA genes in breast cancer, there have been reports of biallelic mutations in Australia, Italy, Denmark, Spain, Korea, and other Caucasian populations [119]. Mutations in FANCM, ATM, FANCE, and PALB2 contribute to locus heterogeneity, besides BRCA1/2 [120]. Cases harboring biallelic and double heterozygosity are listed in Table 6. The study performed in Colombia by Cock et al. found cases caused by locus heterogeneity involving BRCA1, MSH6, RAD51, PMS2, PALB2, BRCA2, and SMAD4 [92]. Interestingly, patients with double heterozygosity presented a familial cancer history and were diagnosed at early ages (before 40 years) [92]. In addition, HBC cases caused by biallelic variants in BRCA1/2 genes affecting young patients were reported in Brazil, Mexico, and Venezuela [63,87,114]. Notoriously, a 12-year old Argentinian patient with a triple-negative breast tumor was double heterozygous for BRCA1 and BRCA2 variants classified as benign, although she also carried some other non-reported variants [101]. Studies in communities like those described here may help to define the clinical phenotypes of HBC cases caused by locus heterogeneity.
Table 6.
Biallelic and locus heterogeneity mutations reported in LA breast cancer cases.
| Patient ID | Gene 1 | Gene 2 | Age Onset | Country | Family History | Subtype | Reference |
|---|---|---|---|---|---|---|---|
| NA | BRCA1: c.1674del (pathogenic) |
MSH6: c.2419G>A (uncertain significance) |
NA | Colombia | YES | NA | [92] |
| NA | BRCA1: c.1674del (pathogenic) |
PMS2: c.2395C>T (uncertain significance); RAD51C: c.492T>G (uncertain significance) |
NA | Colombia | YES | NA | [92] |
| 15 | PALB2: c.1240C>T (pathogenic) |
PMS2: c.241G>A (uncertain significance) |
36 | Colombia | YES | ER+, HER2-, invasive ductal carcinoma | [92] |
| 8 | BRCA2: c.5616-5620del (not reported) |
SMAD4: c.677C>T (conflicting interpretations) |
35 | Colombia | YES | HR+, HER2- invasive ductal carcinoma | [92] |
| NA | BRCA1: c.4357+1G>T (pathogenic) | BRCA2: c.6405_6409delCTTAA (pathogenic) | 38 | Brazil | NA | ipsilateral BC | [63] |
| NA | BRCA1: LGR (deletion of exons 4–6) |
BRCA2: c.9004G>A (conflicting interpretation) |
43 | Brazil | NA | NA | [63] |
| NA | BRCA2: c.8878C>T (pathogenic), c.9699_9702delTATG (pathogenic) |
52 | Brazil | NA | NA | [63] | |
| CM001 | BRCA1: c.1129_1135insA (not reported), c.4063_4065delAAT (conflicting interpretations) | 37 | Venezuela | YES | ER+, PR+ | [114] | |
| CM055 | BRCA1: c.1129_1135insA (not reported), c.4063_4065delAAT (conflicting interpretations) | 48 | Venezuela | YES | ER-, PR- | [114] | |
| CM031 | BRCA2: c.1282T>C (not reported), c.3479G>A (conflicting interpretations) |
49 | Venezuela | YES | NA | [114] | |
| 5 | BRCA2: c.865A>C (benign), c.2971A>G(benign), c.8851G>A (benign) |
33 | Mexico | NA | ductal | [87] | |
| 12 | BRCA1: c.2245G>T (uncertain significance) |
BRCA2: p.Ile3412Val (benign) | 34 | Mexico | NA | ductal | [87] |
| 7 | BRCA1: c.442-34C>T (benign) |
BRCA2: c.865A>C (benign), c.2971A>G (benign) |
34 | Mexico | NA | ductal | [87] |
| 17 | BRCA1: c.3548A>G (benign), c.442-34C>T (benign) |
30 | Mexico | YES | ER+, PR+, HER2- | [89] | |
| A11 | BRCA1: c.4308T>C (benign), c.442-34C (not reported), c.5152+66G>A (benign), c.548-58delT (benign) |
BRCA2: c.426+67A>C (not reported), c.426-89T>C (benign), c.7435+53C>T (benign) | 12 | Argentina | YES | TNBC, Secretory carcinoma | [101] |
| A17 | BRCA1: c.4308T>C (benign), c.5152+66G>A (benign), c.548-58delT (benign) |
25 | Argentina | NO | PR+, ER+, HER2+, infiltrating ductal carcinoma | [101] | |
| A18 | BRCA1: c.442-34T>C (not reported) |
BRCA2: c.7469T>C (benign), c.681+56C>T (benign), c.7242A>G (benign) |
21 | Argentina | NO | ER+, PR+ HER2-, infiltrating lobular carcinoma | [101] |
() Pathogenicity: ClinVar; NA: Not Available; LGR: Large Genomic Rearrangements.
Nowadays, a plethora of commercial testing panels are available including “Breast Next” from Ambry Genetics, “OncoGeneDx” from GeneDx, “My Risk” from Myriad Genetics, and others. These panels along with NGS and exome analysis provide a large amount of data yet to be analyzed for breast cancer risk and its management [121]. According to the NCCN guidelines, genetic evaluation of genes like BRCA1/2, ATM, CDH1, CHEK2, NBN, NF1, and PALB2 is recommended for HBC prevention. Other genes associated to breast cancer risk included in the NCCN guidelines are BARD1, BRIP1, MSH2, MLH1, MSH6, PMS2, RAD51C, and RAD51D, but they are not considered for breast cancer management and assessment [16]. The growing number of genes and variants associated with HBC is a challenge to the standardized systems used for clinical testing, including the re-evaluation of the variants of unknown significance (VUS). VUS are not rare, for example, there are thousands of VUS reported for BRCA1/2 genes [122,123]. The increased number of VUS in BRCA genes correlates with the discovery of new variants in non-Caucasian ethnic groups. In individuals of European ancestry across the United States, VUS account for approximately 5–6% of the variants reported in clinical tests, and up to 21% in patients with African-American ancestry. Recent studies show a higher rate of VUS in BRCA1/2 for non-Caucasians (36%) than in Caucasians (27%) [124]. Testing laboratories in Europe estimate that up to 15% of alterations in BRCA1/2 are VUS [123]. Furthermore, reported variants in non-BRCA genes increase the complexity of pathogenicity classification and genetic counseling.
Information on non-BRCA gene variants in LA is scarce due to the low coverage for genetic testing in the health systems of the region, among other reasons [92,125]. This hinders the identification of new variants, their evaluation for clinical significance, and the risk management assessment.
5. Therapy Recommendation for DNA Repair Related Genes
DNA double-strand breaks (DSB) are among the first procedures that take place in cancer formation and progression because of endogenous and exogenous factors [126]. With DSB, two main mechanisms may be activated during the repair process, HR and non-homologous end joining (NHEJ) [127]. Most of the non-BRCA genes frequently reported in breast cancer participate in different DNA repair pathways. Reported DSB repair variants in breast cancer comprise PALB2, NBN, RAD51, ATM, CHEK2, ATR, RAD50, and WRN [128]. Likewise, reported variants include genes participating in mismatch repair (MMR) such as MSH2, MSH6, PMS2, and MLH1 [129] and genes participating in the Fanconi Anemia repair pathway like FANCM, FANCI, FANCB, FANCC, FANCL [130]. In addition to these pathways, variants in XPC, ERCC1, ERCC2 and ERCC3 genes relate to nucleotide excision repair. Variants in XRCC1 and MUTYH participate in base excision repair [131].
DNA repair genes display high mutation incidence in cancer, when DNA repair pathways are compromised, mutation rate arises because alternative error-prone repair pathways are used by the cell [11,132]. The study of the repair pathway mechanisms has identified new targets for therapy that might be useful in some types of cancer. For instance, PARP inhibitors such as Olaparib are used for ovarian cancer treatment based on the concept of synthetic lethality and are currently being studied in breast, prostate and gastrointestinal cancers. Besides PARP, there are other key components with potential for targeted therapy; ATR and ATM are major targets for inhibition as well as CHEK1/2 and DNA-PKs. For instance, M6620, the first inhibitor of ATR has been tested and ATM inhibitors such as M3541 are currently in clinical trials [133,134].
A different therapy approach that displays promising results in cancer treatment is immunotherapy with PD-1 and PD-L1 inhibitors [30,135]. High expression of PD-L1 on tumor cells or tumor-infiltrating lymphocytes (TILs) results in exhaustion of T cells and an attenuated tumor-specific immunity that promotes tumor progression [136]. Diverse studies show a possible relationship between altered DNA repair pathways that increase the mutational burden and immunotherapy response. For example, colorectal cancer patients with altered MMR pathways display high microsatellite instability, expression of PD-L1, as CD3+, CD8+ TILs and tumor-associated macrophages located at invasive fronts of the tumor. The mutational burden was associated with mutations in ATM, MMR deficiency, and therefore loss of expression in MLH1, MSH2, MSH6, and PMS2 [33]. In addition, patients with tumor DNA repair deficiencies such as POLE and POLD mutations, are considered good candidates for checkpoint immunotherapy [137,138]. In non-small cell lung cancer (NSCLC), a study by Chae et al. [28] showed that tumors with altered HR genes, MMR genes or POLE contained higher mutational load than tumors with wildtype DNA repair genes. These tumors also contained higher infiltration of T cells and other cells that perform anti-tumor activity. The best treatment responses were observed in patients with high mutational burden with PD-1 inhibitors. The group of better responder patients displayed a neoantigen load and mutations in POLE, POLD1, and MSH2. Based on the premise that DNA repair loss results in elevated anti-tumor immune response, improved clinical outcomes were observed in patients with DNA repair gene mutations. Therefore, mutations in DNA repair genes in lung cancer were linked to increased TILs as CD4+ and CD8+ in the tumor. For patients with higher mutational burden, there is a greater likelihood of the formation of immunogenic epitopes expressed only in cancerous cells [28]. Similarly, in high-grade ovarian cancer altered BRCA1/2 results in a higher mutational load, therefore, tumors harbor more tumor-specific neoantigens, increased TILs including CD3+ and CD8+ and PD-1 and PD-L1 expression [29,136]. Strickland et al. found that tumors with altered HR repair show higher neoantigens along with improved overall survival. They inferred that high grade serous ovarian cancer with mutations in BRCA1/2 may be more sensitive to immune checkpoint inhibitors PD-1 and PD-L1 in comparison with tumors proficient in HR repair [29].
Breast cancer patients with compromised DNA repair mechanisms display high-risk tumor characteristics such as changes in cell morphology that promote invasion [139]. When HR is compromised, alternative error-prone DNA repair pathways are used and thus there is a chance that errors will occur, such as indels in the cell [140,141]. There are frequently mutated DNA repair genes in breast cancer and their analysis is fundamental to determine the tumor phenotype and clarify if a high mutational load is due to deficient DNA repair performance. PARP inhibitors are approved for some breast cancer cases, Olaparib was approved by the FDA for germline BRCA-mutated metastatic breast cancer on January 12, 2018 [142]. Similarly, Talazoparib was approved for germline BRCA-mutated HER2-negative locally advanced or metastatic breast cancer by the FDA in October, 2018 [143]. Moreover, several studies in Clinical Trials (https://clinicaltrials.gov/ct2/home) are focused on DNA repair genes. For instance, the study NCT03495544 focuses on the association between germline DNA repair genes mutations and PD-L1 expression level in breast cancer. Studies for PARP inhibitors such as Rucaparib have been completed, studies for Niraparib are still recruiting for Phase I and Phase III trials, and BMN673 is being tested in advanced breast cancer. On the other hand, immunotherapy studies in breast cancer like the one conducted by Schmid et al. [144] for triple-negative breast cancer (TNBC) showed that the combination of PD-L1 inhibitor Atezolizumab with nab-paclitaxel resulted in improvement in overall survival with a median of 9.5-month (HR 0.62, 95% CI 0.45–0.86) in patients with PD-L1 positive immune infiltration [144]. Another combined therapy for TNBC of Pembrolizumab an anti-PD-1 with chemotherapy increased the pathological complete response rates [145]. The potential for immunotherapy in breast cancer is promising, suggesting combination therapies for future clinical trials [146].
Even though novel cancer treatment strategies are appearing, in Latin America the availability of these therapies is challenging [147]. Over the last five years, about 64% of medicine newly released to the market was sold exclusively in the US, 24% of these new drugs were sold in Western Europe and 7% in Japan. Therefore, the remaining 5% is distributed in the rest of the world [147]. Between 2009 and 2013, 37 new cancer drugs were launched worldwide and only 17 of them are available in Mexico and 10 in Brazil [148], and access to high-cost cancer drugs is a barrier in the LA region [149]. In LA countries, use of novel drugs differs widely by insurance type, therefore, one promising solution to improve access to these therapies is participation in clinical research [148,149]. For breast cancer, chemotherapy with anthracyclines is accepted in the region, however, targeted therapy drugs such as Trastuzumab is not accessible to all patients. Restrictions to access new drugs leaves patients with few therapeutic alternatives, disease progression, and consequently, poor outcomes [150]. Therefore, one option for new drugs to be available in LA countries is through participation in clinical trials. Some of these countries are currently participating in clinical trials that are focused on PARP inhibitors and immune checkpoint inhibitors. Velaparib, Talazoparib, and Olaparib are PARP inhibitors and currently, active clinical trials NCT01506609, NCT02595905, and NCT02163694 are testing Velaparib in combination with Carboplatin, Paclitaxel, and Cisplatin in breast cancer patients harboring BRCA mutations in Argentina, Brazil, Chile, Colombia, Puerto Rico, Mexico. Similarly, a phase 3 study NCT01945775 in Brazil is currently testing Talazoparib in patients with metastatic breast cancer and BRCA mutations. In addition, Olaparib is currently being tested in metastatic breast cancer patients with germline BRCA1/2 mutation in Peru and Mexico in a phase 3 study (NCT02000622). Also, some immunotherapy agents are currently in clinical trials in Latin America countries. Pembrolizumab, a PD-1 inhibitor, is being tested (NCT02447003) in metastatic TNBC in Puerto Rico. Pembrolizumab in combination with chemotherapy (NCT03036488) is being tested in Colombia and Brazil. Clinical trial NCT03797326 is studying Pembrolizumab in combination with Lenvatinib in Chilean TNBC patients. Clinical trial NCT03725059 is currently recruiting patients in Brazil and Colombia to test neoadjuvant chemotherapy in combination with Pembrolizumab in early stage ER positive, HER2 negative breast cancer. The PD-1 inhibitor Nivolumab is being tested in combination with Daratumab in TNBC patients in Puerto Rico. Atezolizumab a PD-L1 inhibitor, is being tested as an immunotherapy strategy in phase 3 clinical trial NCT02425891 in combination with Nab-Paclitaxel in metastatic TNBC in Mexico, Colombia, Brazil, Argentina, and Chile. Similarly, clinical trials NCT03498716, NCT03197935, and NCT03125902 are recruiting TNBC patients in Mexico, Peru, Brazil, and Argentina to test Atezolizumab in combination with chemotherapy, Anthracyclines, Taxanes, Paclitaxel.
Based on the reported mutation data for LA countries, we consider that breast cancer patients might benefit from novel targeted therapies like PARP-inhibitors and immunotherapy. In the same way, patients with germline mutations in DNA repair genes like PALB2, NBN, RAD51, ATM, CHEK2, ATR, and RAD50, might benefit from PD-L1 inhibitors and PARP inhibitors. In Latin America countries, germline mutations in BRCA and non-BRCA genes have been reported mostly in patients with early onset, advanced disease or TNBC. With this in mind, PARP inhibitors and immunotherapy might be a good strategy for breast cancer patients harboring these previously mentioned mutations. Consequently, further studies focusing on DNA repair gene mutations and their role as novel predictive markers are needed for immunotherapy response and targeted therapy in the DNA damage response (DDR) pathway [28]. We hypothesize that breast cancer patients in Latin America that have mutations in the DNA repair pathway could benefit from these kinds of therapies.
In this study, several BRCA1/2 variants that are considered susceptible to PARP inhibitors were found according to the JAX Clinical Knowledgebase (JAX-CKB) (https://ckb.jax.org) and the Clinical Interpretations of Variants in Cancer database (https://civicdb.org/home). In these databases, seventeen variants in each BRCA1 and BCR2 genes are considered to be sensitive to PARP inhibitors due to loss of function or predicted loss of function. These variants were reported in some LA countries (Argentina, Brazil, Chile, Colombia, Costa Rica, Mexico, Peru, Uruguay, and Venezuela). Interestingly, some variants such as c.211A>G and c.68_69delAG in BRCA1 were each reported in six countries (Argentina, Brazil, Chile, Colombia, Mexico, Peru, and Uruguay); and for BRCA2, variant c.5946delT was observed in five countries (Argentina, Brazil, Chile, Costa Rica, and Peru).
For non-BRCA genes like ATM, PARP inhibitors are suggested for inactivating mutations or loss of function variants; in the same way as for BARD1, CHEK2, MSH2, NBN, and PALB2 genes. Consequently, we consider that patients in different LA countries harboring these variants could benefit from PARP inhibitors therapies.
In this work, even though rare variants are often reported and counted as mutations, most of them have not been tested in functional analysis. Herein, variants that have been reported in at least two countries for breast cancer are included, suggesting that they may have biological significance.
6. Conclusions
Understanding germline mutations in BRCA and non-BRCA genes in Latin American communities is necessary to improve screening strategies and to implement and develop viable precision medicine practices. This study shows that more information and analyses are required to define the prevalence of gene variants involved in HBC in LA, to define their pathogenicity and for reclassifying VUS and variants of conflicting interpretation in this population. This information will facilitate the implementation of HBC screening programs and targeted therapies in Latin America, particularly of those treatments that address DNA repair mechanisms.
The importance of more breast cancer studies, including non-BRCA genes, is to analyze the DNA repair capacity status for a better understanding of the relationship between DNA repair and breast cancer tumor aggressivity, potential biomarkers for prognosis along with immunotherapy recommendations and possible novel targets in DDR. Therefore, patients with mutations in DNA repair genes might also be candidates for targeted therapy in order to improve their outcome. Future studies focusing on non-BRCA genes, mainly DNA repair genes, will be of great benefit for LA breast cancer patients.
7. Take Home Messages
BRCA1/2 are the most analyzed and studied genes in LA countries, few studies report non-BRCA gene status in breast cancer.
In addition to BRCA1/2, non-BRCA genes provide information about the DNA repair capacity status.
Targeted therapy such as immunotherapy and mainly PARP inhibitors are focused on DNA repair gene status for better response.
Studies focusing on non-BRCA genes are needed in LA countries.
Acknowledgments
This work was supported by Tecnologico de Monterrey, Escuela de Medicina y Ciencias de la Salud, as well as a Ph.D. scholarship granted to U.-J.L.K. (CVU #883312) by CONACyT (Mexican Council for Science and Technology).
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-4425/10/10/786/s1, Figure S1: BRCA1/2 and non-BRCA gene variants reported from breast cancer cases in LA countries, Table S1: Frequent variants in non-BRCA genes in LA and their pathogenic classification.
Author Contributions
Investigation and writing—original draft, U.-J.L.K.; writing—review and editing R.-M.A.; writing—review and editing M.-L.E.; resources A.D.; resources V.-G.C.; visualization and supervision, O.-L.R.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries: Global cancer statistics 2018. CA Cancer J. Clin. 2018 doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.El Tannouri R., Albuisson E., Jonveaux P., Luporsi E. Is there a genetic anticipation in breast and/or ovarian cancer families with the germline c.3481_3491del11 mutation? Fam. Cancer. 2018;17:5–14. doi: 10.1007/s10689-017-9999-4. [DOI] [PubMed] [Google Scholar]
- 3.Cragun D., Weidner A., Kechik J., Pal T. Genetic testing across young hispanic and non-hispanic white breast cancer survivors: Facilitators, barriers, and awareness of the genetic information nondiscrimination act. Genet. Test. Mol. Biomark. 2019;23:75–83. doi: 10.1089/gtmb.2018.0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kwong A., Chen J., Shin V.Y. A new paradigm of genetic testing for hereditary breast/ovarian cancers. Hong Kong Med. J. 2016 doi: 10.12809/hkmj154634. [DOI] [PubMed] [Google Scholar]
- 5.Miki Y., Swensen J., Shattuck-Eidens D., Futreal P., Harshman K., Tavtigian S., Liu Q., Cochran C., Bennett L., Ding W., et al. A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science. 1994;266:66–71. doi: 10.1126/science.7545954. [DOI] [PubMed] [Google Scholar]
- 6.Wooster R., Bignell G., Lancaster J., Swift S., Seal S., Mangion J., Collins N., Gregory S., Gumbs C., Micklem G. Identification of the breast cancer susceptibility gene BRCA2. Nature. 1995;378:789–792. doi: 10.1038/378789a0. [DOI] [PubMed] [Google Scholar]
- 7.Friedman L.S., Ostermeyer E.A., Szabo C.I., Dowd P., Lynch E.D., Rowell S.E., King M.C. Confirmation of BRCA1 by analysis of germline mutations linked to breast and ovarian cancer in ten families. Nat. Genet. 1994;8:399–404. doi: 10.1038/ng1294-399. [DOI] [PubMed] [Google Scholar]
- 8.Grynberg M., Dagher Hayeck B., Papanikolaou E.G., Sifer C., Sermondade N., Sonigo C. BRCA1/2 gene mutations do not affect the capacity of oocytes from breast cancer candidates for fertility preservation to mature in vitro. Hum. Reprod. Oxf. Engl. 2019;34:374–379. doi: 10.1093/humrep/dey358. [DOI] [PubMed] [Google Scholar]
- 9.Walsh C.S. Two decades beyond BRCA1/2: Homologous recombination, hereditary cancer risk and a target for ovarian cancer therapy. Gynecol. Oncol. 2015;137:343–350. doi: 10.1016/j.ygyno.2015.02.017. [DOI] [PubMed] [Google Scholar]
- 10.Foulkes W.D. Inherited susceptibility to common cancers. N. Engl. J. Med. 2008;359:2143–2153. doi: 10.1056/NEJMra0802968. [DOI] [PubMed] [Google Scholar]
- 11.Gómez-Flores-Ramos L., Castro-Sanchez A., Peña-Curiel O., Mohar-Betancourt A. Molecular biology in young women with breast cancer: From tumor gene expression to DNA mutations. Rev. Investig. Clínica. 2017;69 doi: 10.24875/ric.17002225. [DOI] [PubMed] [Google Scholar]
- 12.Economopoulou P., Dimitriadis G., Psyrri A. Beyond BRCA: New hereditary breast cancer susceptibility genes. Cancer Treat. Rev. 2015;41:1–8. doi: 10.1016/j.ctrv.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 13.Daza-Martin M., Starowicz K., Jamshad M., Tye S., Ronson G.E., MacKay H.L., Chauhan A.S., Walker A.K., Stone H.R., Beesley J.F.J., et al. Isomerization of BRCA1–BARD1 promotes replication fork protection. Nature. 2019:1. doi: 10.1038/s41586-019-1363-4. [DOI] [PubMed] [Google Scholar]
- 14.Postel-Vinay S., Vanhecke E., Olaussen K.A., Lord C.J., Ashworth A., Soria J.-C. The potential of exploiting DNA-repair defects for optimizing lung cancer treatment. Nat. Rev. Clin. Oncol. 2012;9:144–155. doi: 10.1038/nrclinonc.2012.3. [DOI] [PubMed] [Google Scholar]
- 15.Dietlein F., Thelen L., Reinhardt H.C. Cancer-specific defects in DNA repair pathways as targets for personalized therapeutic approaches. Trends Genet. 2014;30:326–339. doi: 10.1016/j.tig.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 16.Daly M.B., Pilarski R., Berry M., Buys S.S., Farmer M., Friedman S., Garber J.E., Kauff N.D., Khan S., Klein C., et al. NCCN guidelines insights: Genetic/familial high-risk assessment: Breast and ovarian, version 2.2017. J. Natl. Compr. Cancer Netw. 2017;15:9–20. doi: 10.6004/jnccn.2017.0003. [DOI] [PubMed] [Google Scholar]
- 17.Liu Q., Yao S., Zhao H., Hu Q., Kwan M.L., Roh J.M., Ambrosone C.B., Kushi L.H., Liu S., Zhu Q. Early-onset triple-negative breast cancer in multiracial/ethnic populations: Distinct trends of prevalence of truncation mutations. Cancer Med. 2019 doi: 10.1002/cam4.2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chavarri-Guerra Y., Blazer K.R., Weitzel J.N. Genetic cancer risk assessment for breast cancer in Latin America. Rev. Investig. Clín. 2017;69 doi: 10.24875/RIC.17002195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moreno-Estrada A., Gravel S., Zakharia F., McCauley J.L., Byrnes J.K., Gignoux C.R., Ortiz-Tello P.A., Martínez R.J., Hedges D.J., Morris R.W., et al. Reconstructing the population genetic history of the Caribbean. PLoS Genet. 2013;9:e1003925. doi: 10.1371/journal.pgen.1003925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zavala V.A., Serrano-Gomez S.J., Dutil J., Fejerman L. Genetic epidemiology of breast cancer in Latin America. Genes. 2019;10:153. doi: 10.3390/genes10020153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Homburger J.R., Moreno-Estrada A., Gignoux C.R., Nelson D., Sanchez E., Ortiz-Tello P., Pons-Estel B.A., Acevedo-Vasquez E., Miranda P., Langefeld C.D., et al. Genomic insights into the ancestry and demographic history of South America. PLoS Genet. 2015;11:e1005602. doi: 10.1371/journal.pgen.1005602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Asakawa H., Koizumi H., Koike A., Takahashi M., Wu W., Iwase H., Fukuda M., Ohta T. Prediction of breast cancer sensitivity to neoadjuvant chemotherapy based on status of DNA damage repair proteins. Breast Cancer Res. 2010;12 doi: 10.1186/bcr2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meindl A., Ditsch N., Kast K., Rhiem K., Schmutzler R.K. Hereditary breast and ovarian cancer. Dtsch. Aerzteblatt Online. 2011;275:1885–1892. doi: 10.3238/arztebl.2011.0323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yin L., Liu Y., Peng Y., Peng Y., Yu X., Gao Y., Yuan B., Zhu Q., Cao T., He L., et al. PARP inhibitor veliparib and HDAC inhibitor SAHA synergistically co-target the UHRF1/BRCA1 DNA damage repair complex in prostate cancer cells. J. Exp. Clin. Cancer Res. 2018;37 doi: 10.1186/s13046-018-0810-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Annunziata C.M., Bates S.E. PARP inhibitors in BRCA1/BRCA2 germline mutation carriers with ovarian and breast cancer. F1000 Biol. Rep. 2010;2 doi: 10.3410/B2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sullivan-Reed K., Bolton-Gillespie E., Dasgupta Y., Langer S., Siciliano M., Nieborowska-Skorska M., Hanamshet K., Belyaeva E.A., Bernhardy A.J., Lee J., et al. Simultaneous targeting of PARP1 and RAD52 triggers dual synthetic lethality in BRCA-deficient tumor cells. Cell Rep. 2018;23:3127–3136. doi: 10.1016/j.celrep.2018.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brown J.S., O’Carrigan B., Jackson S.P., Yap T.A. Targeting DNA repair in cancer: Beyond PARP inhibitors. Cancer Discov. 2017;7:20–37. doi: 10.1158/2159-8290.CD-16-0860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chae Y.K., Anker J.F., Bais P., Namburi S., Giles F.J., Chuang J.H. Mutations in DNA repair genes are associated with increased neo-antigen load and activated T cell infiltration in lung adenocarcinoma. Oncotarget. 2018;9 doi: 10.18632/oncotarget.23742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Strickland K.C., Howitt B.E., Shukla S.A., Rodig S., Ritterhouse L.L., Liu J.F., Garber J.E., Chowdhury D., Wu C.J., D’Andrea A.D., et al. Association and prognostic significance of BRCA1/2-mutation status with neoantigen load, number of tumor-infiltrating lymphocytes and expression of PD-1/PD-L1 in high grade serous ovarian cancer. Oncotarget. 2016;7 doi: 10.18632/oncotarget.7277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gong J., Chehrazi-Raffle A., Reddi S., Salgia R. Development of PD-1 and PD-L1 inhibitors as a form of cancer immunotherapy: A comprehensive review of registration trials and future considerations. J. Immunother. Cancer. 2018;6 doi: 10.1186/s40425-018-0316-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Overman M.J., Lonardi S., Wong K.Y.M., Lenz H.-J., Gelsomino F., Aglietta M., Morse M.A., Van Cutsem E., McDermott R., Hill A., et al. Durable clinical benefit with nivolumab plus ipilimumab in DNA mismatch repair–deficient/microsatellite instability–high metastatic colorectal cancer. J. Clin. Oncol. 2018;36:773–779. doi: 10.1200/JCO.2017.76.9901. [DOI] [PubMed] [Google Scholar]
- 32.Yi D., Xu L., Luo J., You X., Huang T., Zi Y., Li X., Wang R., Zhong Z., Tang X., et al. Germline TP53 and MSH6 mutations implicated in sporadic triple-negative breast cancer (TNBC): A preliminary study. Hum. Genom. 2019;13 doi: 10.1186/s40246-018-0186-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hodges T.R., Ott M., Xiu J., Gatalica Z., Swensen J., Zhou S., Huse J.T., de Groot J., Li S., Overwijk W.W., et al. Mutational burden, immune checkpoint expression, and mismatch repair in glioma: Implications for immune checkpoint immunotherapy. Neuro Oncol. 2017;19:1047–1057. doi: 10.1093/neuonc/nox026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brandalize A.P.C., Schüler-Faccini L., Hoffmann J.-S., Caleffi M., Cazaux C., Ashton-Prolla P. A DNA repair variant in POLQ (c.-1060A > G) is associated to hereditary breast cancer patients: A case–control study. BMC Cancer. 2014;14 doi: 10.1186/1471-2407-14-850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de Souza Timoteo A.R., Gonçalves A.É.M.M., Sales L.A.P., Albuquerque B.M., de Souza J.E.S., de Moura P.C.P., de Aquino M.A.A., Agnez-Lima L.F., Lajus T.B.P. A portrait of germline mutation in Brazilian at-risk for hereditary breast cancer. Breast Cancer Res. Treat. 2018;172:637–646. doi: 10.1007/s10549-018-4938-0. [DOI] [PubMed] [Google Scholar]
- 36.Assumpção J.G., Seidinger A.L., Mastellaro M.J., Ribeiro R.C., Zambetti G.P., Ganti R., Srivastava K., Shurtleff S., Pei D., Zeferino L.C., et al. Association of the germline TP53R337H mutation with breast cancer in southern Brazil. BMC Cancer. 2008;8 doi: 10.1186/1471-2407-8-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lourenço J.J., Vargas F.R., Bines J., Santos E.M., Lasmar C.A.P., Costa C.H., Teixeira E.M.B., Maia M.C.M., Coura F., Silva C.H.D., et al. BRCA1 mutations in Brazilian patients. Genet. Mol. Biol. 2004;27:500–504. doi: 10.1590/S1415-47572004000400006. [DOI] [Google Scholar]
- 38.Leistner-Segal Analysis of the R72P polymorphism of the TP53 gene in patients with invasive ductal breast carcinoma. Mol. Med. Rep. 2009;2 doi: 10.3892/mmr_00000174. [DOI] [PubMed] [Google Scholar]
- 39.Abud J., Prolla J.C., Koehler-Santos P., Ashton-Prolla P. CHEK2 1100DELC germline mutation: A frequency study in hereditary breast and colon cancer Brazilian families. Arq. Gastroenterol. 2012;49:273–278. doi: 10.1590/S0004-28032012000400008. [DOI] [PubMed] [Google Scholar]
- 40.Torrezan G.T., de Almeida F.G.D.S.R., de Figueiredo Barros B.D., de Paula C.A., Valieris R., de Souza J.E.S., Ramalho R.F., da Silva F.C.C., Ferreira E.N., et al. Complex landscape of germline variants in Brazilian patients with hereditary and early onset breast cancer. Front. Genet. 2018;9 doi: 10.3389/fgene.2018.00161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ewald I.P., Cossio S.L., Palmero E.I., Pinheiro M., de Oliveira Nascimento I.L., Machado T.M.B., Sandes K.A., Toralles B., Garicochea B., Izetti P., et al. BRCA1 and BRCA2 rearrangements in Brazilian individuals with hereditary breast and ovarian cancer syndrome. Genet. Mol. Biol. 2016;39:223–231. doi: 10.1590/1678-4685-gmb-2014-0350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Carraro D.M., Koike Folgueira M.A.A., Garcia Lisboa B.C., Ribeiro Olivieri E.H., Vitorino Krepischi A.C., de Carvalho A.F., de Carvalho Mota L.D., Puga R.D., do Socorro Maciel M., Michelli R.A.D., et al. Comprehensive analysis of BRCA1, BRCA2 and TP53 germline mutation and tumor characterization: a portrait of early-onset breast cancer in Brazil. PLoS ONE. 2013;8:e57581. doi: 10.1371/journal.pone.0057581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dufloth R.M., Costa S., Schmitt F., Zeferino L.C. DNA repair gene polymorphisms and susceptibility to familial breast cancer in a group of patients from Campinas, Brazil. Genet. Mol. Res. 2005:13. [PubMed] [Google Scholar]
- 44.Cipriano N.M., de Brito A.M., de Oliveira E.S., de Faria F.C., Lemos S., Rodrigues A.N., de Oliveira Lopes D., dos Santos L.L. Mutation screening of TP53, CHEK2 and BRCA genes in patients at high risk for hereditary breast and ovarian cancer (HBOC) in Brazil. Breast Cancer. 2018 doi: 10.1007/s12282-018-00938-z. [DOI] [PubMed] [Google Scholar]
- 45.Brianese R.C., de Mello Nakamura K.D., Ramalho R.F., de Figueiredo Barros B.D., e Ferreira E.N., da Cruz Formiga M.N., de Andrade V.P., de Lima V.C.C., Carraro D.M. BRCA1 deficiency is a recurrent event in early-onset triple-negative breast cancer: A comprehensive analysis of germline mutations and somatic promoter methylation. Breast Cancer Res. Treat. 2018;167:803–814. doi: 10.1007/s10549-017-4552-6. [DOI] [PubMed] [Google Scholar]
- 46.Felix G.E., Abe-Sandes C., Machado-Lopes T.M., Bomfim T.F., Guindalini R.S.C., Santos V.C.S., Meyer L., Oliveira P.C., Cláudio Neiva J., Meyer R., et al. Germline mutations in BRCA1, BRCA2, CHEK2 and TP53 in patients at high-risk for HBOC: Characterizing a northeast Brazilian population. Hum. Genome Var. 2014;1 doi: 10.1038/hgv.2014.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gomes M.C., Kotsopoulos J., de Almeida G.L., Costa M.M., Vieira R., de AG Filho F., Pitombo M.B., Leal P.R., Royer R., Zhang P., et al. The R337H mutation in TP53 and breast cancer in Brazil. Hered. Cancer Clin. Practi. 2012;10 doi: 10.1186/1897-4287-10-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cury N.M., Ferraz V.E., Silva W.A. TP53 p.R337H prevalence in a series of Brazilian hereditary breast cancer families. Hered. Cancer Clin. Practi. 2014;12:8. doi: 10.1186/1897-4287-12-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hahn E.C., Bittar C.M., Vianna F.S.L., Netto C.B.O., Biazús J.V., Cericatto R., Cavalheiro J.A., de Melo M.P., Menke C.H., Rabin E., et al. TP53 p.Arg337His germline mutation prevalence in Southern Brazil: Further evidence for mutation testing in young breast cancer patients. PLoS ONE. 2018;13:e0209934. doi: 10.1371/journal.pone.0209934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dillenburg C.V., Bandeira I.C., Tubino T.V., Rossato L.G., Dias E.S., Bittelbrunn A.C., Leistner-Segal S. Prevalence of 185delAG and 5382insC mutations in BRCA1, and 6174delT in BRCA2 in women of Ashkenazi Jewish origin in southern Brazil. Genet. Mol. Biol. 2012;35:599–602. doi: 10.1590/S1415-47572012000400009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Esteves V.F., Thuler L.C.S., Amêndola L.C., Koifman R.J., Koifman S., Frankel P.P., Vieira R.J.S. Prevalence of BRCA1 and BRCA2 gene mutations in families with medium and high risk of breast and ovarian cancer in Brazil. Braz. J. Med. Biol. Res. 2009;42:453–457. doi: 10.1590/S0100-879X2009000500009. [DOI] [PubMed] [Google Scholar]
- 52.Gomes M.C.B., Costa M.M., Borojevic R., Monteiro A.N.A., Vieira R., Koifman S., Koifman R.J., Li S., Royer R., Zhang S., et al. Prevalence of BRCA1 and BRCA2 mutations in breast cancer patients from Brazil. Breast Cancer Res. Treat. 2007;103:349–353. doi: 10.1007/s10549-006-9378-6. [DOI] [PubMed] [Google Scholar]
- 53.Fernandes G.C., Michelli R.A.D., Galvão H.C.R., Paula A.E., Pereira R., Andrade C.E., Felicio P.S., Souza C.P., Mendes D.R.P., Volc S., et al. Prevalence of BRCA1/BRCA2 mutations in a Brazilian population sample at-risk for hereditary breast cancer and characterization of its genetic ancestry. Oncotarget. 2016;7 doi: 10.18632/oncotarget.12610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ewald I.P., Izetti P., Vargas F.R., Moreira M.A., Moreira A.S., Moreira-Filho C.A., Cunha D.R., Hamaguchi S., Camey S.A., Schmidt A., et al. Prevalence of the BRCA1 founder mutation c.5266dupin Brazilian individuals at-risk for the hereditary breast and ovarian cancer syndrome. Hered. Cancer Clin. Practi. 2011;9 doi: 10.1186/1897-4287-9-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Giacomazzi J., Graudenz M.S., Osorio C.A.B.T., Koehler-Santos P., Palmero E.I., Zagonel-Oliveira M., Michelli R.A.D., Neto C.S., Fernandes G.C., Achatz M.I.W.S., et al. Prevalence of the TP53 p.R337H mutation in breast cancer patients in Brazil. PLoS ONE. 2014;9:e99893. doi: 10.1371/journal.pone.0099893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Palmero E.I., Alemar B., Schüler-Faccini L., Hainaut P., Moreira-Filho C.A., Ewald I.P., dos Santos P.K., Ribeiro P.L.I., de Oliveira Netto C.B., Calvez-Kelm F.L., et al. Screening for germline BRCA1, BRCA2, TP53 and CHEK2 mutations in families at-risk for hereditary breast cancer identified in a population-based study from Southern Brazil. Genet. Mol. Biol. 2016;39:210–222. doi: 10.1590/1678-4685-gmb-2014-0363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Andrade K.C., Santiago K.M., Fortes F.P., Mambelli L.I., Nóbrega A.F., Achatz M.I. Early-onset breast cancer patients in the South and Southeast of Brazil should be tested for the TP53 p.R337H mutation. Genet. Mol. Biol. 2016;39:199–202. doi: 10.1590/1678-4685-gmb-2014-0343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.da Costa E.C.B., Vargas F.R., Moreira A.S., Lourenço J.J., Caleffi M., Ashton-Prolla P., Martins Moreira M.A.M. Founder effect of the BRCA1 5382insC mutation in Brazilian patients with hereditary breast ovary cancer syndrome. Cancer Genet. Cytogenet. 2008;184:62–66. doi: 10.1016/j.cancergencyto.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 59.de Oliveira E.S., Soares B.L., Lemos S., Rosa R.C.A., Rodrigues A.N., Barbosa L.A., de Oliveira Lopes D., dos Santos L.L. Screening of the BRCA1 gene in Brazilian patients with breast and/or ovarian cancer via high-resolution melting reaction analysis. Fam. Cancer. 2016;15:173–181. doi: 10.1007/s10689-015-9858-0. [DOI] [PubMed] [Google Scholar]
- 60.Palmero E.I., Carraro D.M., Alemar B., Moreira M.A.M., Ribeiro-dos-Santos Â., Abe-Sandes K., Galvão H.C.R., Reis R.M., de Pádua Souza C., Campacci N., et al. The germline mutational landscape of BRCA1 and BRCA2 in Brazil. Sci. Rep. 2018;8 doi: 10.1038/s41598-018-27315-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Almeida B.C., Kleine J.P.F.O., Camargo-Kosugi C.M., Lisboa M.R., França C.N., França J.P., Silva I.D.C.G. Analysis of polymorphisms in codons 11, 72 and 248 of TP53 in Brazilian women with breast cancer. Genet. Mol. Res. 2016;15 doi: 10.4238/gmr.15017055. [DOI] [PubMed] [Google Scholar]
- 62.Rodrigues M.S., Machado C.A., Pagnoncelli D., Avvad E., da Paixão J.C., Gallo C.V.D.M. TP53 and XRCC1 polymorphisms and breast cancer prognosis: A case-case study. Clinics. 2011;66:1097–1100. doi: 10.1590/S1807-59322011000600030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Alemar B., Gregório C., Herzog J., Matzenbacher Bittar C., Brinckmann Oliveira Netto C., Artigalas O., Schwartz I.V.D., Coffa J., Alves Camey S., Weitzel J., et al. BRCA1 and BRCA2 mutational profile and prevalence in hereditary breast and ovarian cancer (HBOC) probands from Southern Brazil: Are international testing criteria appropriate for this specific population? PLoS ONE. 2017;12:e0187630. doi: 10.1371/journal.pone.0187630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.de Souza Timoteo A.R., Albuquerque B.M., Moura P.P.P., de Oliveira Ramos C.C., Agnez-Lima L.F., Walsh T., King M.-C., Lajus T.B.P. Identification of a new BRCA2 large genomic deletion associated with high risk male breast cancer. Hered. Cancer Clin. Practi. 2015;13 doi: 10.1186/s13053-014-0022-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Silva F.C., Lisboa B.C., Figueiredo M.C., Torrezan G.T., Santos É.M., Krepischi A.C., Rossi B.M., Achatz M.I., Carraro D.M. Hereditary breast and ovarian cancer: Assessment of point mutations and copy number variations in Brazilian patients. BMC Med. Genet. 2014;15:55. doi: 10.1186/1471-2350-15-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tapia T., Sanchez A., Vallejos M., Alvarez C., Moraga M., Smalley S., Camus M., Alvarez M., Carvallo P. ATM allelic variants associated to hereditary breast cancer in 94 Chilean women: Susceptibility or ethnic influences? Breast Cancer Res. Treat. 2008;107:281–288. doi: 10.1007/s10549-007-9544-5. [DOI] [PubMed] [Google Scholar]
- 67.Alvarez C., Tapia T., Perez-Moreno E., Gajardo-Meneses P., Ruiz C., Rios M., Missarelli C., Silva M., Cruz A., Matamala L., et al. BRCA1 and BRCA2 founder mutations account for 78% of germline carriers among hereditary breast cancer families in Chile. Oncotarget. 2017;8 doi: 10.18632/oncotarget.18815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jara L., Ampuero S., Santibáñez E., Seccia L., Rodríguez J., Bustamante M., Martínez V., Catenaccio A., Lay-Son G., Blanco R., et al. BRCA1 and BRCA2 mutations in a South American population. Cancer Genet. Cytogenet. 2006;166:36–45. doi: 10.1016/j.cancergencyto.2005.08.019. [DOI] [PubMed] [Google Scholar]
- 69.González-Hormazábal P., Bravo T., Blanco R., Valenzuela C.Y., Gómez F., Waugh E., Peralta O., Ortuzar W., Reyes J.M., Jara L. Association of common ATM variants with familial breast cancer in a South American population. BMC Cancer. 2008;8:117. doi: 10.1186/1471-2407-8-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gallardo M., Silva A., Rubio L., Alvarez C., Torrealba C., Salinas M., Tapia T., Faundez P., Palma L., Riccio M.E., et al. Incidence of BRCA1 and BRCA2 mutations in 54 Chilean families with breast/ovarian cancer, genotype–phenotype correlations. Breast Cancer Res. Treat. 2006;95:81–87. doi: 10.1007/s10549-005-9047-1. [DOI] [PubMed] [Google Scholar]
- 71.Leyton Y., Gonzalez-Hormazabal P., Blanco R., Bravo T., Fernandez-Ramires R., Morales S., Landeros N., Reyes J.M., Peralta O., Tapia J.C., et al. Association of PALB2 sequence variants with the risk of familial and early-onset breast cancer in a South-American population. BMC Cancer. 2015;15 doi: 10.1186/s12885-015-1033-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gonzalez-Hormazabal P., Gutierrez-Enriquez S., Gaete D., Reyes J.M., Peralta O., Waugh E., Gomez F., Margarit S., Bravo T., Blanco R., et al. Spectrum of BRCA1/2 point mutations and genomic rearrangements in high-risk breast/ovarian cancer Chilean families. Breast Cancer Res. Treat. 2011;126:705–716. doi: 10.1007/s10549-010-1170-y. [DOI] [PubMed] [Google Scholar]
- 73.Jara L., Ampuero S., Santibáñez E., Seccia L., Rodríguez J., Bustamante M., Lay-Son G., Ojeda J.M., Reyes J.M., Blanco R. Molecular analysis of the eighteen most frequent mutations in the BRCA1 gene in 63 Chilean breast cancer families. Biol. Res. 2004;37 doi: 10.4067/S0716-97602004000300011. [DOI] [PubMed] [Google Scholar]
- 74.Jara L., Acevedo M.L., Blanco R., Castro V.G., Bravo T., Gómez F., Waugh E., Peralta O., Cabrera E., Reyes J.M., et al. RAD51 135G>C polymorphism and risk of familial breast cancer in a South American population. Cancer Genet. Cytogenet. 2007;178:65–69. doi: 10.1016/j.cancergencyto.2007.05.024. [DOI] [PubMed] [Google Scholar]
- 75.Gonzalez-Hormazabal P., Reyes J.M., Blanco R., Bravo T., Carrera I., Peralta O., Gomez F., Waugh E., Margarit S., Ibañez G., et al. The BARD1 Cys557Ser variant and risk of familial breast cancer in a South-American population. Mol. Biol. Rep. 2012;39:8091–8098. doi: 10.1007/s11033-012-1656-2. [DOI] [PubMed] [Google Scholar]
- 76.Jara L., Dubois K., Gaete D., de Mayo T., Ratkevicius N., Bravo T., Margarit S., Blanco R., Gómez F., Waugh E., et al. Variants in DNA double-strand break repair genes and risk of familial breast cancer in a South American population. Breast Cancer Res. Treat. 2010;122:813–822. doi: 10.1007/s10549-009-0709-2. [DOI] [PubMed] [Google Scholar]
- 77.Jara L., Ampuero Ll S., Seccia L., Bustamante M., Blanco R., Santibáñez E., Reyes J.M., Ojeda J.M. Frecuencia de la mutación 185delAG en el gen BRCA1 en mujeres chilenas sanas con antecedentes familiares de cáncer de mama. Rev. Médica Chile. 2002;130:1113–1123. doi: 10.4067/S0034-98872002001000005. [DOI] [PubMed] [Google Scholar]
- 78.Gallardo C.M., Faúndez J.P., Cruz A., Rodríguez M., Alvarez Z.M., Carvallo SQ P. Determinación de una mutación en el gen BRCA1 en una familia que presenta cáncer de mama hereditario. Rev. Médica Chile. 2004;132 doi: 10.4067/S0034-98872004000200010. [DOI] [PubMed] [Google Scholar]
- 79.González-Hormazábal P., Castro V.G., Blanco R., Gómez F., Peralta O., Waugh E., Bravo T., Reyes J.M., Jara L. Absence of CHEK2 1100delC mutation in familial breast cancer cases from a South American population. Breast Cancer Res. Treat. 2008;110:543–545. doi: 10.1007/s10549-007-9743-0. [DOI] [PubMed] [Google Scholar]
- 80.Fernández-Lopez J.C., Romero-Córdoba S., Rebollar-Vega R., Alfaro-Ruiz L.A., Jiménez-Morales S., Beltrán-Anaya F., Arellano-Llamas R., Cedro-Tanda A., Rios-Romero M., Ramirez-Florencio M., et al. Population and breast cancer patients’ analysis reveals the diversity of genomic variation of the BRCA genes in the Mexican population. Hum. Genom. 2019;13 doi: 10.1186/s40246-018-0188-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Quezada Urban R., Díaz Velásquez C., Gitler R., Rojo Castillo M., Sirota Toporek M., Figueroa Morales A., Moreno García O., García Esquivel L., Torres Mejía G., Dean M., et al. Comprehensive analysis of germline variants in mexican patients with hereditary breast and ovarian cancer susceptibility. Cancers. 2018;10:361. doi: 10.3390/cancers10100361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Calderón-Zúñiga F.D.C., Ocampo-Gómez G., López-Márquez F.C., Recio-Vega R., Serrano-Gallardo L.B., Ruiz-Flores P. ATM polymorphisms IVS24-9delT, IVS38-8T>C, and 5557G>A in Mexican women with familial and/or early-onset breast cancer. Salud Publica Mexico. 2014;56:206–212. doi: 10.21149/spm.v56i2.7336. [DOI] [PubMed] [Google Scholar]
- 83.Ruiz-Flores P., Sinilnikova O.M., Badzioch M., Calderon-Garcidueñas A.L., Chopin S., Fabrice O., González-Guerrero J.F., Szabo C., Lenoir G., Goldgar D.E., et al. BRCA1 and BRCA2 mutation analysis of early-onset and familial breast cancer cases in Mexico: Mutations in brief. Hum. Mutat. 2002;20:474–475. doi: 10.1002/humu.9084. [DOI] [PubMed] [Google Scholar]
- 84.Villarreal-Garza C., Weitzel J.N., Llacuachaqui M., Sifuentes E., Magallanes-Hoyos M.C., Gallardo L., Alvarez-Gómez R.M., Herzog J., Castillo D., Royer R., et al. The prevalence of BRCA1 and BRCA2 mutations among young Mexican women with triple-negative breast cancer. Breast Cancer Res. Treat. 2015;150:389–394. doi: 10.1007/s10549-015-3312-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gómez-Díaz B., De La Luz Ayala-Madrigal M., Gutiérrez-Angulo M., Valle-Solis A.E., Linares-González L.M., González-Guzmán R., Cruz-Guillén D., Cedeño-Garcidueñas A.L., Canto P., López-Hernández L.B. Analysis of ERCC1 and ERCC2 gene variants in osteosarcoma, colorectal and breast cancer. Oncol. Lett. 2015;9:1657–1661. doi: 10.3892/ol.2015.2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ziv E. Genetics of breast cancer: Applications to the Mexican population. Salud Pública México. 2011;53:5. [PubMed] [Google Scholar]
- 87.Calderón-Garcidueñas A.L., Ruiz-Flores P., Cerda-Flores R.M., Barrera-Saldaña H.A. Clinical follow up of Mexican women with early onset of breast cancer and mutations in the BRCA1 and BRCA2 genes. Salud Pública México. 2005;47:110–115. doi: 10.1590/s0036-36342005000200004. [DOI] [PubMed] [Google Scholar]
- 88.Villarreal-Garza C., Alvarez-Gómez R.M., Pérez-Plasencia C., Herrera L.A., Herzog J., Castillo D., Mohar A., Castro C., Gallardo L.N., Gallardo D., et al. Significant clinical impact of recurrent BRCA1 and BRCA2 mutations in Mexico: Recurrent BRCA mutations in Mexico. Cancer. 2015;121:372–378. doi: 10.1002/cncr.29058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Vaca-Paniagua F., Alvarez-Gomez R.M., Fragoso-Ontiveros V., Vidal-Millan S., Herrera L.A., Cantú D., Bargallo-Rocha E., Mohar A., López-Camarillo C., Pérez-Plasencia C. Full-exon pyrosequencing screening of BRCA germline mutations in Mexican women with inherited breast and ovarian cancer. PLoS ONE. 2012;7:e37432. doi: 10.1371/journal.pone.0037432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Torres-Mejia G., Royer R., Llacuachaqui M., Akbari M.R., Giuliano A.R., Martinez-Matsushita L., Angeles-Llerenas A., Ortega-Olvera C., Ziv E., Lazcano-Ponce E., et al. Recurrent BRCA1 and BRCA2 mutations in Mexican women with breast cancer. Cancer Epidemiol. Prev. Biomark. 2015;24:498–505. doi: 10.1158/1055-9965.EPI-13-0980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Macías-Gómez N.M., Peralta-Leal V., Meza-Espinoza J.P., Gutiérrez-Angulo M., Durán-González J., Ramírez-González J.M., Gaspar-Del Toro A., Norberto-Rodríguez A., Leal-Ugarte E. Polymorphisms of the XRCC1 gene and breast cancer risk in the Mexican population. Fam. Cancer. 2015;14:349–354. doi: 10.1007/s10689-015-9787-y. [DOI] [PubMed] [Google Scholar]
- 92.Cock-Rada A.M., Ossa C.A., Garcia H.I., Gomez L.R. A multi-gene panel study in hereditary breast and ovarian cancer in Colombia. Fam. Cancer. 2018;17:23–30. doi: 10.1007/s10689-017-0004-z. [DOI] [PubMed] [Google Scholar]
- 93.Hernández J.E.L., Llacuachaqui M., Palacio G.V., Figueroa J.D., Madrid J., Lema M., Royer R., Li S., Larson G., Weitzel J.N., et al. Prevalence of BRCA1 and BRCA2 mutations in unselected breast cancer patients from Medellín, Colombia. Hered. Cancer Clin. Pract. 2014;12 doi: 10.1186/1897-4287-12-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Torres D., Bermejo J.L., Rashid M.U., Briceño I., Gil F., Beltran A., Ariza V., Hamann U. Prevalence and penetrance of BRCA1 and BRCA2 germline mutations in Colombian breast cancer patients. Sci. Rep. 2017;7 doi: 10.1038/s41598-017-05056-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Briceño-Balcázar I., Gómez-Gutiérrez A., Díaz-Dussán N.A., Noguera-Santamaría M.C., Díaz D., Casas-Gómez M.C. Mutational spectrum in breast cancer associated BRCA1 and BRCA2 genes in Colombia. Colomb. Méd. 2017;48:6. [PMC free article] [PubMed] [Google Scholar]
- 96.Torres D., Rashid M.U., Gil F., Umana A., Ramelli G., Robledo J.F., Tawil M., Torregrosa L., Briceno I., Hamann U. High proportion of BRCA1/2 founder mutations in Hispanic breast/ovarian cancer families from Colombia. Breast Cancer Res. Treat. 2007;103:225–232. doi: 10.1007/s10549-006-9370-1. [DOI] [PubMed] [Google Scholar]
- 97.Llinás-Quintero N., Cabrera-Florez E., Mendoza-Fandiño G., Matute-Turizo G., Vasquez-Trespalacios E.M., Gallón-Villegas L.J. Synchronous ovarian and breast cancers with a novel variant in BRCA2 gene: A case report. Case Rep. Oncol. Med. 2019;2019:1–5. doi: 10.1155/2019/6958952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Solano A.R., Aceto G.M., Delettieres D., Veschi S., Neuman M.I., Alonso E., Chialina S., Chacón R.D., Renato M.-C., Podestá E.J. BRCA1 And BRCA2 analysis of Argentinean breast/ovarian cancer patients selected for age and family history highlights a role for novel mutations of putative south-American origin. SpringerPlus. 2012;1 doi: 10.1186/2193-1801-1-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Solano A.R., Liria N.C., Jalil F.S., Faggionato D.M., Mele P.G., Mampel A., Cardoso F.C., Podesta E.J. BRCA1 and BRCA2 mutations other than the founder alleles among Ashkenazi Jewish in the population of Argentina. Front. Oncol. 2018;8 doi: 10.3389/fonc.2018.00323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Solano A.R., Cardoso F.C., Romano V., Perazzo F., Bas C., Recondo G., Santillan F.B., Gonzalez E., Abalo E., Viniegra M., et al. Spectrum of BRCA1/2 variants in 940 patients from Argentina including novel, deleterious and recurrent germline mutations: Impact on healthcare and clinical practice. Oncotarget. 2017;8 doi: 10.18632/oncotarget.10814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Aceto G.M., Solano A.R., Neuman M.I., Veschi S., Morgano A., Malatesta S., Chacon R.D., Pupareli C., Lombardi M., Battista P., et al. High-risk human papilloma virus infection, tumor pathophenotypes, and BRCA1/2 and TP53 status in juvenile breast cancer. Breast Cancer Res. Treat. 2010;122:671–683. doi: 10.1007/s10549-009-0596-6. [DOI] [PubMed] [Google Scholar]
- 102.Abugattas J., Llacuachaqui M., Allende Y.S., Velásquez A.A., Velarde R., Cotrina J., Garcés M., León M., Calderón G., de la Cruz M., et al. Prevalence of BRCA1 and BRCA2 mutations in unselected breast cancer patients from Peru: Prevalence of BRCA1/2 mutations in unselected breast cancer patients from Peru. Clin. Genet. 2015;88:371–375. doi: 10.1111/cge.12505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Buleje J., Guevara-Fujita M., Acosta O., Huaman F.D.P., Danos P., Murillo A., Pinto J.A., Araujo J.M., Aguilar A., Ponce J., et al. Mutational analysis of BRCA1 and BRCA2 genes in Peruvian families with hereditary breast and ovarian cancer. Mol. Genet. Genom. Med. 2017;5:481–494. doi: 10.1002/mgg3.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.González-Rivera M., Lobo M., López-Tarruella S., Jerez Y., del Monte-Millán M., Massarrah T., Ramos-Medina R., Ocaña I., Picornell A., Garzón S.S., et al. Frequency of germline DNA genetic findings in an unselected prospective cohort of triple-negative breast cancer patients participating in a platinum-based neoadjuvant chemotherapy trial. Breast Cancer Res. Treat. 2016;156:507–515. doi: 10.1007/s10549-016-3792-1. [DOI] [PubMed] [Google Scholar]
- 105.Diaz-Zabala H., Ortiz A., Garland L., Jones K., Perez C., Mora E., Arroyo N., Oleksyk T., Echenique M., Matta J., et al. A Recurrent BRCA2 Mutation explains the majority of hereditary breast and ovarian cancer syndrome cases in Puerto Rico. Cancers. 2018;10:419. doi: 10.3390/cancers10110419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Dutil J., Colon-Colon J.L., Matta J.L., Sutphen R., Echenique M. Identification of the prevalent BRCA1 and BRCA2 mutations in the female population of Puerto Rico. Cancer Genet. 2012;205:242–248. doi: 10.1016/j.cancergen.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Pérez-Mayoral J., Pacheco-Torres A.L., Morales L., Acosta-Rodríguez H., Matta J.L., Dutil J. Genetic polymorphisms in RAD23B and XPC modulate DNA repair capacity and breast cancer risk in Puerto Rican women: RAD23B polymorphisms in breast cancer risk. Mol. Carcinog. 2013;52:127–138. doi: 10.1002/mc.22056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Delgado L., Fernández G., Grotiuz G., Cataldi S., González A., Lluveras N., Heguaburu M., Fresco R., Lens D., Sabini G., et al. BRCA1 and BRCA2 germline mutations in Uruguayan breast and breast–ovarian cancer families. Identification of novel mutations and unclassified variants. Breast Cancer Res. Treat. 2011;128:211–218. doi: 10.1007/s10549-010-1320-2. [DOI] [PubMed] [Google Scholar]
- 109.Valle D.A.D., Acevedo C., Esperón P., Neffa F., Artagaveytia N., Santander G., Menini M., Vergara B.C., Carusso B.F., Sapone L.M. Cáncer de mama y ovario hereditario en Uruguay: Resultados del screening para mutaciones en genes de susceptibilidad por secuenciación de nueva generación. Rev. Med. Urug. 2017;33:40–52. [Google Scholar]
- 110.Delgado L., Fernández G., González A., Paillerets B.B., Gualco G., Bombled J., Cataldi S., Sabini G., Roca R., Musé I.M. Hereditary breast cancer associated with a germline BRCA2 mutation in identical female twins with similar disease expression. Cancer Genet. Cytogenet. 2002;133:24–28. doi: 10.1016/S0165-4608(01)00541-6. [DOI] [PubMed] [Google Scholar]
- 111.Gutiérrez Espeleta G., Llacuachaqui M., García-Jiménez L., Aguilar Herrera M., Loáiciga Vega K., Ortiz A., Royer R., Li S., Narod S. BRCA1 and BRCA2 mutations among familial breast cancer patients from Costa Rica. Clin. Genet. 2012;82:484–488. doi: 10.1111/j.1399-0004.2011.01774.x. [DOI] [PubMed] [Google Scholar]
- 112.García-Jiménez L., Gutiérrez-Espeleta G., Narod S.A. Epidemiología descriptiva y genética molecular del cáncer de mama hereditario en Costa Rica. Rev. Biol. Trop. 2012;60:1663–1668. doi: 10.15517/rbt.v60i4.2159. [DOI] [PubMed] [Google Scholar]
- 113.Rodriguez R.C., Esperon A.A., Ropero R., Rubio M.C., Rodriguez R., Ortiz R.M., Anta J.J.L., de los Rios M., Carnesolta D., del Olivera M.C., et al. Prevalence of BRCA1 and BRCA2 mutations in breast cancer patients from Cuba. Fam. Cancer. 2008;7:275–279. doi: 10.1007/s10689-008-9187-7. [DOI] [PubMed] [Google Scholar]
- 114.Lara K., Consigliere N., Pérez J., Porco A. BRCA1 and BRCA2mutations in breast cancer patients from Venezuela. Biol. Res. 2012;45:117–130. doi: 10.4067/S0716-97602012000200003. [DOI] [PubMed] [Google Scholar]
- 115.Ashton-Prolla P., Vargas F.R. Prevalence and impact of founder mutations in hereditary breast cancer in Latin America. Genet. Mol. Biol. 2014;37:234–240. doi: 10.1590/S1415-47572014000200009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ossa C.A., Torres D. Founder and recurrent mutations in BRCA1 and BRCA2 genes in Latin American countries: State of the art and literature review. Oncology. 2016;21:832–839. doi: 10.1634/theoncologist.2015-0416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cerami E., Gao J., Dogrusoz U., Gross B.E., Sumer S.O., Aksoy B.A., Jacobsen A., Byrne C.J., Heuer M.L., Larsson E., et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2:401–404. doi: 10.1158/2159-8290.CD-12-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gao J., Aksoy B.A., Dogrusoz U., Dresdner G., Gross B., Sumer S.O., Sun Y., Jacobsen A., Sinha R., Larsson E., et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6:pl1. doi: 10.1126/scisignal.2004088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Palmirotta R., Lovero D., Stucci L., Silvestris E., Quaresmini D., Cardascia A., Silvestris F. Double heterozygosity for BRCA1 pathogenic variant and BRCA2 polymorphic stop codon K3326X: A case report in a southern Italian family. Int. J. Mol. Sci. 2018;19:285. doi: 10.3390/ijms19010285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Riaz N., Blecua P., Lim R.S., Shen R., Higginson D.S., Weinhold N., Norton L., Weigelt B., Powell S.N., Reis-Filho J.S. Pan-cancer analysis of bi-allelic alterations in homologous recombination DNA repair genes. Nat. Commun. 2017;8 doi: 10.1038/s41467-017-00921-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Easton D.F., Pharoah P.D.P., Antoniou A.C., Tischkowitz M., Tavtigian S.V., Nathanson K.L., Devilee P., Meindl A., Couch F.J., Southey M., et al. Gene-panel sequencing and the prediction of breast-cancer risk. N. Engl. J. Med. 2015;372:2243–2257. doi: 10.1056/NEJMsr1501341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Vink G.R., van Asperen C.J., Devilee P., Breuning M.H., Bakker E. Unclassified variants in disease-causing genes: Nonuniformity of genetic testing and counselling, a proposal for guidelines. Eur. J. Hum. Genet. 2005;13:525–527. doi: 10.1038/sj.ejhg.5201379. [DOI] [PubMed] [Google Scholar]
- 123.Lindor N.M., Goldgar D.E., Tavtigian S.V., Plon S.E., Couch F.J. BRCA1/2 sequence variants of uncertain significance: A primer for providers to assist in discussions and in medical management. Oncology. 2013;18:518–524. doi: 10.1634/theoncologist.2012-0452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Caswell-Jin J.L., Gupta T., Hall E., Petrovchich I.M., Mills M.A., Kingham K.E., Koff R., Chun N.M., Levonian P., Lebensohn A.P., et al. Racial/ethnic differences in multiple-gene sequencing results for hereditary cancer risk. Genet. Med. Off. J. Am. Coll. Med. Genet. 2018;20:234–239. doi: 10.1038/gim.2017.96. [DOI] [PubMed] [Google Scholar]
- 125.Jara L., Morales S., de Mayo T., Gonzalez-Hormazabal P., Carrasco V., Godoy R. Mutations in BRCA1, BRCA2 and other breast and ovarian cancer susceptibility genes in Central and South American populations. Biol. Res. 2017:50. doi: 10.1186/s40659-017-0139-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Palla V.-V., Karaolanis G., Katafigiotis I., Anastasiou I., Patapis P., Dimitroulis D., Perrea D. Gamma-H2AX: Can it be established as a classical cancer prognostic factor? Tumor Biol. 2017;39:101042831769593. doi: 10.1177/1010428317695931. [DOI] [PubMed] [Google Scholar]
- 127.Helena J., Joubert A., Grobbelaar S., Nolte E., Nel M., Pepper M., Coetzee M., Mercier A. Deoxyribonucleic acid damage and repair: Capitalizing on our understanding of the mechanisms of maintaining genomic integrity for therapeutic purposes. Int. J. Mol. Sci. 2018;19:1148. doi: 10.3390/ijms19041148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ranjha L., Howard S.M., Cejka P. Main steps in DNA double-strand break repair: An introduction to homologous recombination and related processes. Chromosoma. 2018;127:187–214. doi: 10.1007/s00412-017-0658-1. [DOI] [PubMed] [Google Scholar]
- 129.Liu D., Keijzers G., Rasmussen L.J. DNA mismatch repair and its many roles in eukaryotic cells. Mutat. Res. 2017;773:174–187. doi: 10.1016/j.mrrev.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 130.Gallmeier E., Kern S.E. Targeting Fanconi anemia/BRCA2 pathway defects in cancer: The significance of preclinical pharmacogenomic models. Clin. Cancer Res. 2007;13:4–10. doi: 10.1158/1078-0432.CCR-06-1637. [DOI] [PubMed] [Google Scholar]
- 131.Tulay P., Sengupta S.B. MicroRNA expression and its association with DNA repair in preimplantation embryos. J. Reprod. Dev. 2016;62:225–234. doi: 10.1262/jrd.2015-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Dhawan M., Ryan C.J. BRCAness and prostate cancer: Diagnostic and therapeutic considerations. Prostate Cancer Prostatic Dis. 2018 doi: 10.1038/s41391-018-0069-2. [DOI] [PubMed] [Google Scholar]
- 133.Gavande N.S., VanderVere-Carozza P.S., Hinshaw H.D., Jalal S.I., Sears C.R., Pawelczak K.S., Turchi J.J. DNA repair targeted therapy: The past or future of cancer treatment? Pharm. Ther. 2016;160:65–83. doi: 10.1016/j.pharmthera.2016.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Pilié P.G., Tang C., Mills G.B., Yap T.A. State-of-the-art strategies for targeting the DNA damage response in cancer. Nat. Rev. Clin. Oncol. 2018 doi: 10.1038/s41571-018-0114-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Dine J., Gordon R., Shames Y., Kasler M.K., Barton-Burke M. Immune Checkpoint inhibitors: An innovation in immunotherapy for the treatment and management of patients with cancer. Asia-Pac. J. Oncol. Nurs. 2017;4:127–135. doi: 10.4103/apjon.apjon_4_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Yi M., Jiao D., Xu H., Liu Q., Zhao W., Han X., Wu K. Biomarkers for predicting efficacy of PD-1/PD-L1 inhibitors. Mol. Cancer. 2018;17 doi: 10.1186/s12943-018-0864-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Palles C., Cazier J.-B., Howarth K.M., Domingo E., Jones A.M., Broderick P., Kemp Z., Spain S.L., Almeida E.G., Salguero I., et al. Germline mutations in the proof-reading domains of POLE and POLD1 predispose to colorectal adenomas and carcinomas. Nat. Genet. 2013;45:136–144. doi: 10.1038/ng.2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Marginean E.C., Melosky B. Is there a role for programmed death ligand-1 testing and immunotherapy in colorectal cancer with microsatellite instability? Part I—Colorectal cancer: Microsatellite instability, testing, and clinical implications. Arch. Pathol. Lab. Med. 2018;142:17–25. doi: 10.5858/arpa.2017-0040-RA. [DOI] [PubMed] [Google Scholar]
- 139.Bai F., Chan H.L., Scott A., Smith M.D., Fan C., Herschkowitz J.I., Perou C.M., Livingstone A.S., Robbins D.J., Capobianco A.J., et al. BRCA1 suppresses epithelial-to-mesenchymal transition and stem cell dedifferentiation during mammary and tumor development. Cancer Res. 2014;74:6161–6172. doi: 10.1158/0008-5472.CAN-14-1119. [DOI] [PubMed] [Google Scholar]
- 140.Deniz M., Kaufmann J., Stahl A., Gundelach T., Janni W., Hoffmann I., Keimling M., Hampp S., Ihle M., Wiesmüller L. In vitro model for DNA double-strand break repair analysis in breast cancer reveals cell type–specific associations with age and prognosis. FASEB J. 2016;30:3786–3799. doi: 10.1096/fj.201600453R. [DOI] [PubMed] [Google Scholar]
- 141.Keimling M., Deniz M., Varga D., Stahl A., Schrezenmeier H., Kreienberg R., Hoffmann I., König J., Wiesmüller L. The power of DNA double-strand break (DSB) repair testing to predict breast cancer susceptibility. FASEB J. 2012;26:2094–2104. doi: 10.1096/fj.11-200790. [DOI] [PubMed] [Google Scholar]
- 142.US Food and Drug Administration FDA Approves Olaparib for Germline BRCA-Mutated Metastatic Breast Cancer. [(accessed on 4 July 2019)]; Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-olaparib-germline-brca-mutated-metastatic-breast-cancer.
- 143.US Food and Drug Administration FDA Approves Talazoparib for GBRCAm HER2-Negative Locally Advanced or Metastatic Breast Cancer. [(accessed on 4 July 2019)]; Available online: https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-talazoparib-gbrcam-her2-negative-locally-advanced-or-metastatic-breast-cancer.
- 144.Schmid P., Adams S., Rugo H.S., Schneeweiss A., Barrios C.H., Iwata H., Diéras V., Hegg R., Im S.-A., Wright G.S., et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N. Engl. J. Med. 2018 doi: 10.1056/NEJMoa1809615. [DOI] [PubMed] [Google Scholar]
- 145.Nanda R., Liu M.C., Yau C., Asare S., Hylton N., Veer L.V., Perlmutter J., Wallace A.M., Chien A.J., Forero-Torres A., et al. Pembrolizumab plus standard neoadjuvant therapy for high-risk breast cancer (BC): Results from I-SPY 2. J. Clin. Oncol. 2017;35:506. doi: 10.1200/JCO.2017.35.15_suppl.506. [DOI] [Google Scholar]
- 146.Gilmore E., McCabe N., Kennedy R.D., Parkes E.E. DNA Repair Deficiency in Breast Cancer: Opportunities for Immunotherapy. [(accessed on 4 July 2019)]; doi: 10.1155/2019/4325105. Available online: https://www.hindawi.com/journals/jo/2019/4325105/ [DOI] [PMC free article] [PubMed]
- 147.Barrios C.H., Reinert T., Werutsky G. Access to high-cost drugs for advanced breast cancer in Latin America, particularly trastuzumab. Ecancermedicalscience. 2019;13 doi: 10.3332/ecancer.2019.898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ruiz R., Strasser-Weippl K., Touya D., Vincent C.H., Hernandez-Blanquisett A., Louis J.S., Bukowski A., Goss P.E. Improving access to high-cost cancer drugs in Latin America: Much to be done. Cancer. 2017;123:1313–1323. doi: 10.1002/cncr.30549. [DOI] [PubMed] [Google Scholar]
- 149.Pinto J.A., Pinillos L., Villarreal-Garza C., Morante Z., Villarán M.V., Mejía G., Caglevic C., Aguilar A., Fajardo W., Usuga F., et al. Barriers in Latin America for the management of locally advanced breast cancer. Ecancermedicalscience. 2019;13 doi: 10.3332/ecancer.2019.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Cazap E. Breast Cancer in Latin America: A Map of the Disease in the Region. Am. Soc. Clin. Oncol. Educ. Book. 2018;38:451–456. doi: 10.1200/EDBK_201315. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.