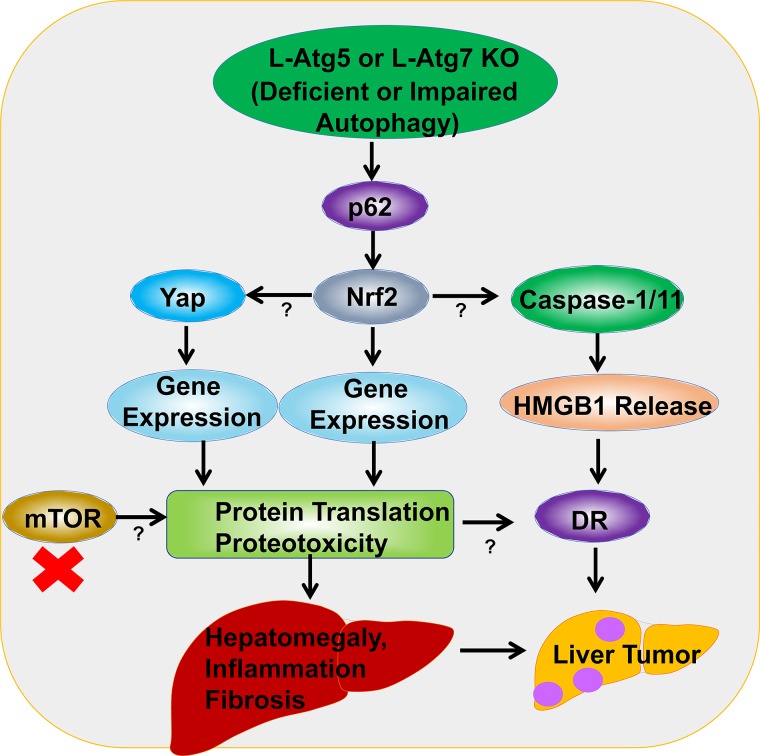
Figure 1.
A proposed model of the molecular events in autophagy deficiency-induced liver pathogenesis. Liver-specific Atg5 (L-Atg5) or L-Atg7 knockout (KO) mice have increased accumulation of hepatic p62 resulting in persistent activation of nuclear factor (erythroid-derived 2)-like 2 (Nrf2). Nrf2 activation activates caspase 1/11 and inflammasome that promotes the release of hepatic high-mobility group box 1 (HMGB1). Nrf2 activation also promotes yes-associated protein (Yap) accumulation and activation in autophagy-deficient livers. However, the exact mechanisms of how Nrf2 activates caspase 1/11 and Yap are unclear. Release of HMGB1 promotes ductular reaction (DR) and liver tumorigenesis without affecting hepatomegaly, liver injury, inflammation, and fibrosis. Nrf2 and Yap upregulate the expression of a large set of their target genes and subsequent protein translation resulting in the accumulation of hepatic proteins and proteotoxicity in autophagy-deficient livers. Accumulation of hepatic proteins and increased proteotoxicity then promote hepatomegaly, inflammation, DR, fibrosis, and tumorigenesis. Inhibition of mTOR may suppress overall protein translation and protein input, which may in turn inhibit proteotoxicity and subsequent liver injury in autophagy-deficient livers.