Figure 10.
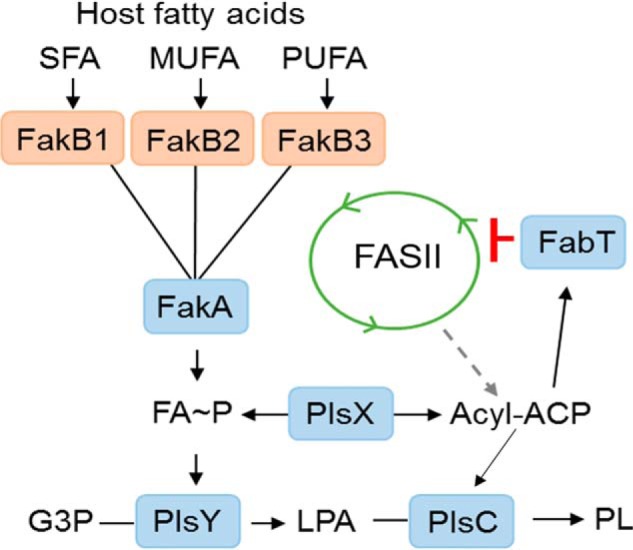
Model for the function of FakB proteins in S. pneumoniae. The de novo phospholipid biosynthetic pathway in S. pneumoniae delivers acyl-ACP generated by the FASII system to PlsX to provide FA∼P to the glycerol phosphate (G3P) acyltransferase (PlsY) to form lysophosphatidic acid (LPA) and initiate phospholipid synthesis. Acyl-ACP is also a substrate for PlsC that completes the formation of phosphatidic acid, the universal precursor to membrane glycerolipids (PL). At the infection site the host provides an environment containing a spectrum of saturated (SFA), monounsaturated (MUFA), and polyunsaturated (PUFA) FA. Each of these types of FA are the preferred ligand for binding to unique FakB-binding proteins that carry the FA to FakA, where they are phosphorylated. The resulting FA∼P is a substrate for PlsY or is converted to acyl-ACP by PlsX to act as substrate for PlsC. The long-chain acyl-ACP binds to the FabT transcriptional regulator, and the complex binds tightly to the promoters within the FASII gene cluster to potently suppresses the expression of the entire biosynthetic gene set and shut off acyl-ACP formation from FASII. The acyl-ACP may also enter FASII and be elongated, although this is a minor pathway due to the strong suppression of the elongation cycle enzymes by FabT–acyl-ACP complex.
