Figure 7.
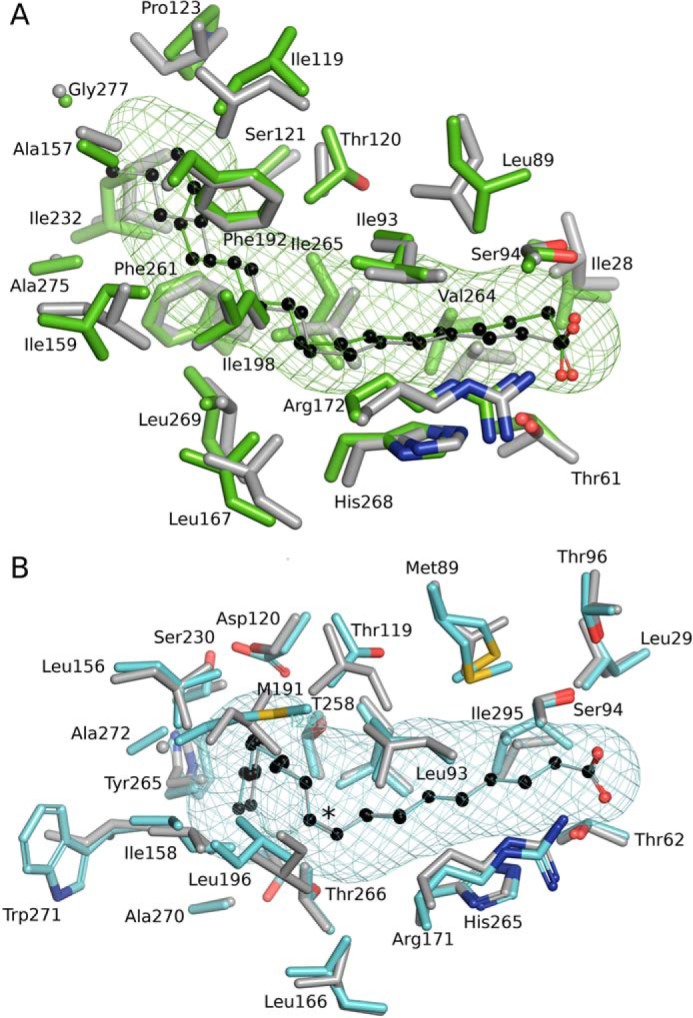
Conservation of the FA-binding pocket shapes in saturated and monounsaturated FA-binding proteins of S. pneumoniae and S. aureus. A, structural alignment of SpFakB1(16:0) (green, PDB ID, 6DKE) and SaFakB1(16:0) (gray, PDB ID, 5UTO) FA-binding tunnels and surrounding residues as aligned with PyMOL. B, SpFakB2(18:1Δ9) (cyan, PDB ID 6DJ6) and SaFakB2(18:1Δ9) (gray, PDB ID, 4X9X). The coordinate alignment was based on the aligned tunnel residues in PyMOL. Asterisk indicates sp2 hybridization of the double bond. The bound FA are depicted as colored sticks with black carbon spheres. The meshes that delineate the hydrophobic cavity within the proteins were computed with CAVER/PyMOL.
