Figure 8.
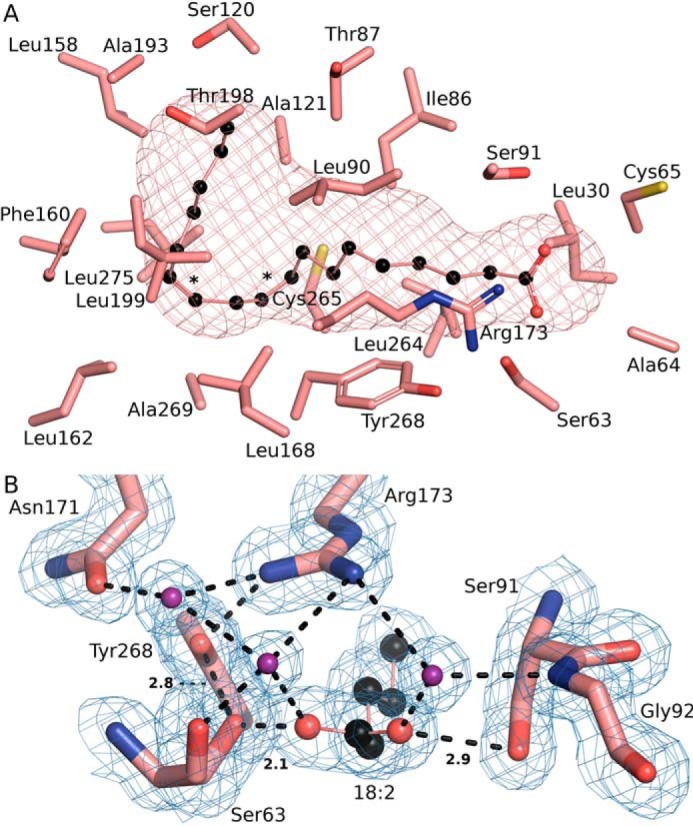
Unique aspects of the SpFakB3 structure. A, view of the SpFakB3 FA-binding tunnel and associated residues as visualized using CAVER to outline the interior volume of FakB3(18:2(Δ9Δ12)) (PDB ID 6CNG) (salmon). FA carbons are black. B, the SpFakB3 Ser-91–FA–Ser-63–Tyr-268 hydrogen bond network that fixes the position of the FA carbonyl in the protein. FA carbons are black, FA and amino acid oxygens are red, and water molecules are colored purple. The blue mesh represents the experimental electron density contoured at 1 σ. Dashed lines indicate hydrogen bonds and the lengths in Å of the three key interactions are shown.
