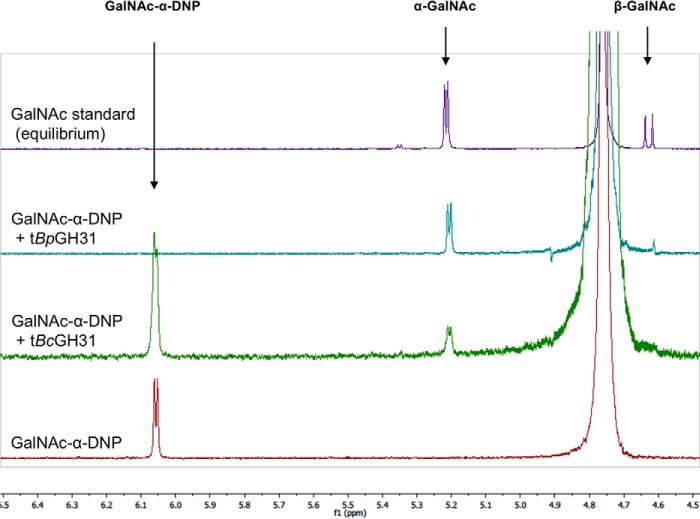
Figure 5.
Determination of the stereochemical outcome of enzymatic hydrolysis at the anomeric center by 1H NMR. The hydrolyses of GalNAc-α-DNP (5 mm) by tBpGH31 and tBcGH31 at room temperature were monitored by 1H NMR. A 1H NMR spectrum of the reaction was obtained after 5 min, and the anomeric stereochemistry of the hydrolysis product was determined. Stereochemistry was determined by observation of the product anomeric hydrogen peak appearing at either 5.2 ppm (α-GalNAc) or 4.6 ppm (β-GalNAc). Only α-GalNAc product was observed following rapid hydrolysis of the starting material, indicating net retention of stereochemistry.