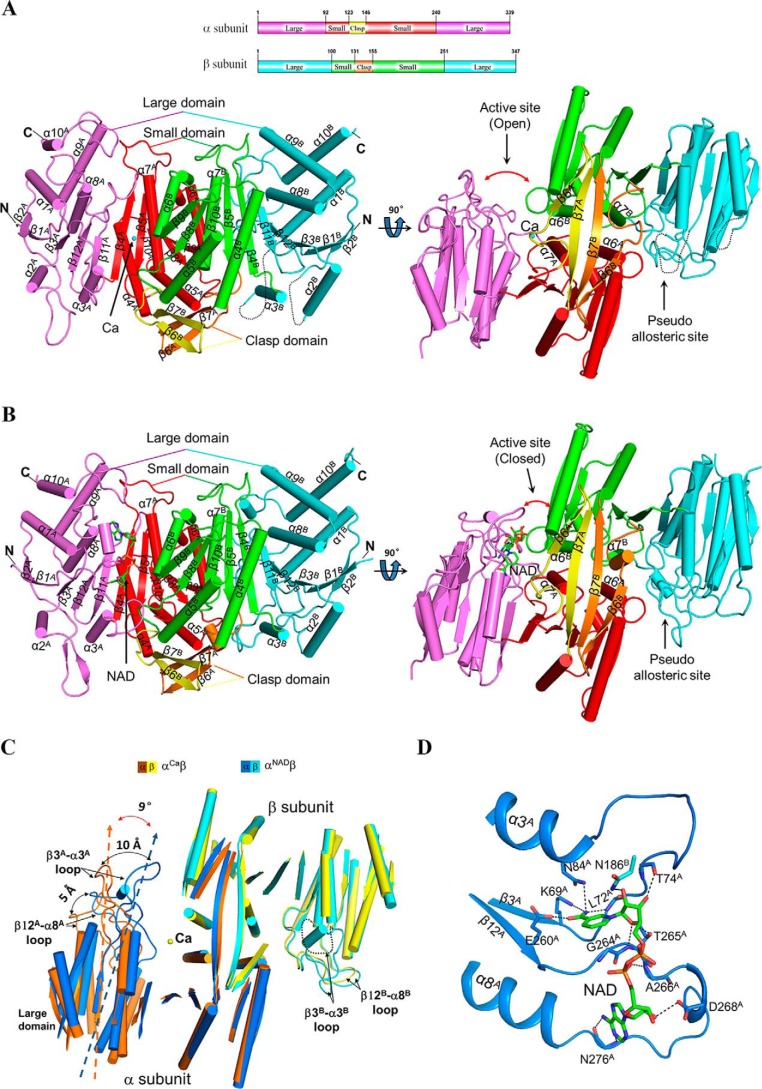
Figure 2.
Overall structure of the αβ heterodimer of human NAD-IDH. A, overall structure of αCaβ in two different orientations. Right, view along the pseudo-2-fold axis of the αβ heterodimer. Left, view perpendicular to the pseudo-2-fold axis of the αβ heterodimer. The color-coding scheme of individual domains of the α and β subunits is shown above. The bound Ca2+ is shown as a sphere. Secondary structure elements of the α and β subunits are labeled with superscripts A and B, respectively. The disordered regions are shown with black dotted lines. B, overall structure of αNADβ in two different orientations. The color-coding scheme is the same as in Fig. 2A. The bound NAD is shown with a ball-and-stick model. C, comparison of αCaβ and αNADβ based on superposition of the β subunits. The color-coding scheme of the α and β subunits of αCaβ and αNADβ is shown above. Upon the NAD binding, the large domain of the α subunit exhibits a notable rotation toward the small domain of the β subunit, and particularly the β3A-α3A loop and the β12A-α8A loop move toward the β subunit by about 10 and 5 Å, respectively. For clarity, only the α-helices and β-strands are shown, and the loops are omitted except for the β12-α8 and β3-α3 loops. D, interactions of NAD with the surrounding residues in αNADβ. The NAD is shown with a ball-and-stick model, and the residues involved in the interactions are shown with side chains and labeled. Hydrogen-bonding interactions are indicated with black dashed lines.