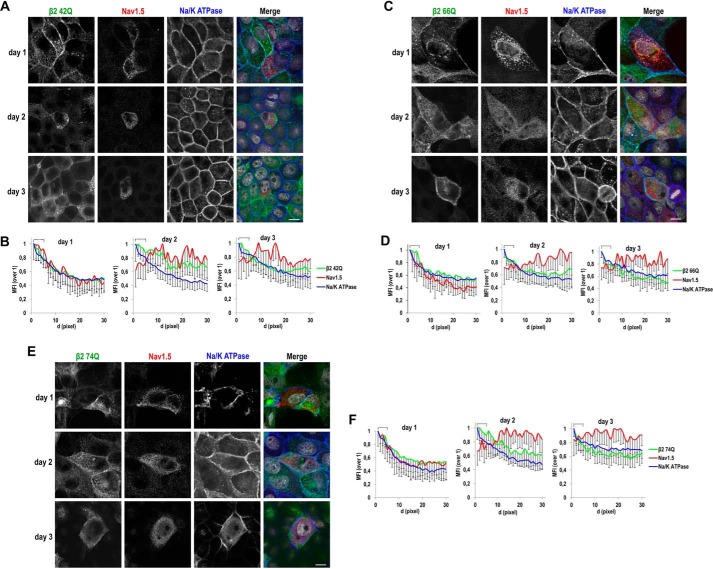
Figure 10.
Single β2 glycosylation mutants can promote surface localization of NaV1.5; analysis over time. MDCK cells stably expressing the indicated single mutant for β2-YFP glycosylation were transiently transfected with the vector SCN5A-FLAG and grown in wells for the specified number of days. Cells were fixed and immunostained with a rabbit polyclonal antibody against NaV1.5 (red) and with a mouse mAb to Na/K-ATPase (blue). Images were obtained by confocal microscopy. In merged images, the YFP-emitted fluorescence is in green and DAPI is in gray. A, C, and E, Representative xy sections (taken at the level where NaV1.5 is mainly found in each case) show some areas of overlap of NaV1.5 with Na/K-ATPase at the cell end, particularly at day 1, in the presence of any of the mutants, while remaining mostly disperse throughout the cell at later time points. Scale bars, 10 μm. Confocal images were analyzed by calculating the MFI along linear segments of 30 pixels in length (d, distance; 0.1 μm/pixel) drawn from the cell end perpendicularly into the cytoplasm. B, D, and F, line charts show MFIs with the first 5 pixels of the segments, equivalent to the plasma membrane region (cell end), marked with a square bracket. The highest MFI levels are at the cell end for Na/K-ATPase and for the different β2 single mutants, all progressively decreasing intracellularly. The profile for NaV1.5 increases at the cell end at day 1 in all cases, remaining comparatively low within this region at later time points. Data are mean ± S.D. (error bars) (number of cells analyzed ≥3, 4 segments/cell).