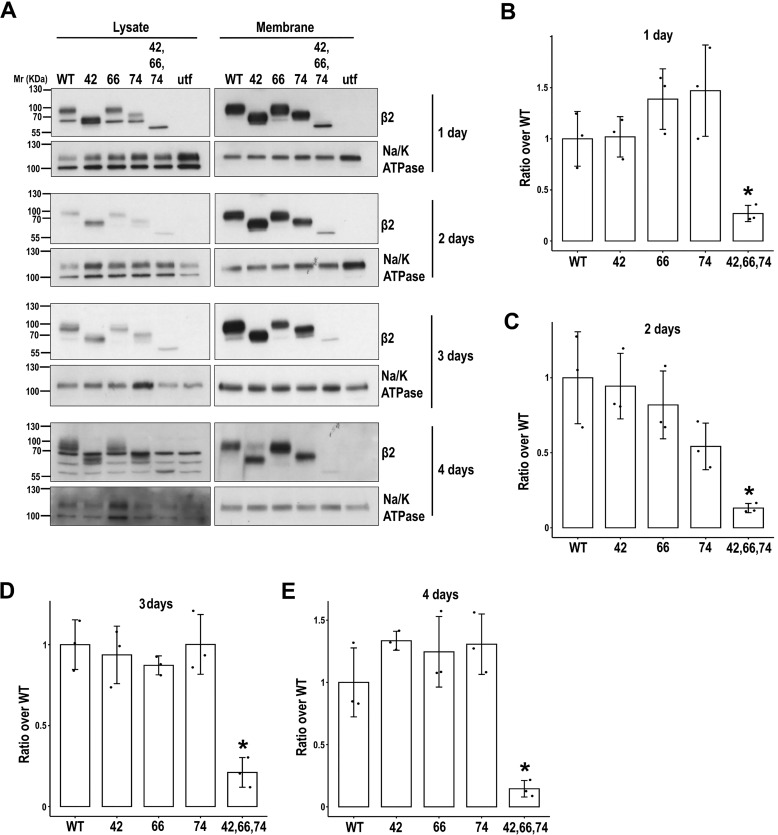
Figure 4.
Defect over time in β2 surface localization due to lack of N-glycosylation. MDCK cells were transiently transfected with the SCN2B-yfp vector to express WT or partially or fully unglycosylated β2 (UNG). Cells were grown in wells for the indicated number of days and surface-biotinylated at 4 °C. Representative Western blots (A) and band quantitation (B–E) show that levels of fully unglycosylated β2 were reduced compared with the WT and partially glycosylated mutants in biotin-NeutrAvidin pulldowns (Membrane). One-way ANOVA with Tukey's HSD post hoc test highlighted these differences, with a few exceptions (B, all p < 0.05, except 42 versus UNG (p = 0.054); C, all p < 0.05 except 42 versus UNG (p = 0.052) and 74 versus UNG (p = 0.189); D, p < 0.002; E, p < 0.005). The same amount of protein was used to process each lysate (∼130 μg), and the corresponding portion (nine-tenths) was subjected to overnight pulldown. Na/K-ATPase was blotted as surface marker to correct for quantitations in pulldowns. Data are mean ± S.D. (error bars) (n ≥ 3). Molecular mass markers are in kDa.