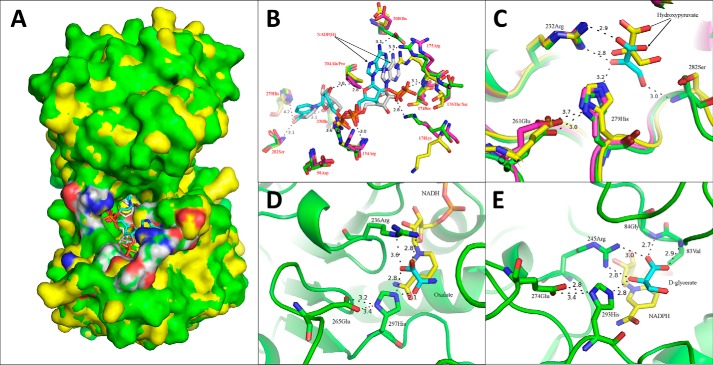
Figure 6.
Co-enzyme docking analyses of Vv2KGR and the two substrate-binding modes of 2KDHs. A, overall positioning of the docked coenzyme NADPH (cyan) in Vv2KGR (green) in superimposition with CbHPR (yellow) complexed with NADPH (gray). The active-site residues are highlighted in white (carbon), blue (nitrogen), and red (oxygen). B, identification of the critical residues responsible for co-enzyme binding in Vv2KGR (green) and alignment with CbHPR (3BAZ (yellow) and 3BA1 (magenta)). The NADPHs for Vv2KGR and 3BAZ are colored cyan and gray, respectively. The potential hydrogen bond interactions with NADPH in Vv2KGR are indicated by black dashed lines with distance labeled. C, identification of the substrate-binding sites of Vv2KGR (green) in superimposition with CbHPR (magenta) and GRHPR (yellow). D and E, substrate-binding mode A (PDB code 2DLD; L. helvveticus d-lactate dehydrogenase bound with NADH (yellow) and oxalate (cyan)) and Mode B (PDB code 2GCG; GRHPR bound with NADPH (yellow) and d-glycerate (cyan)), respectively. The potential hydrogen-bonding interactions are indicated by black dashed lines.