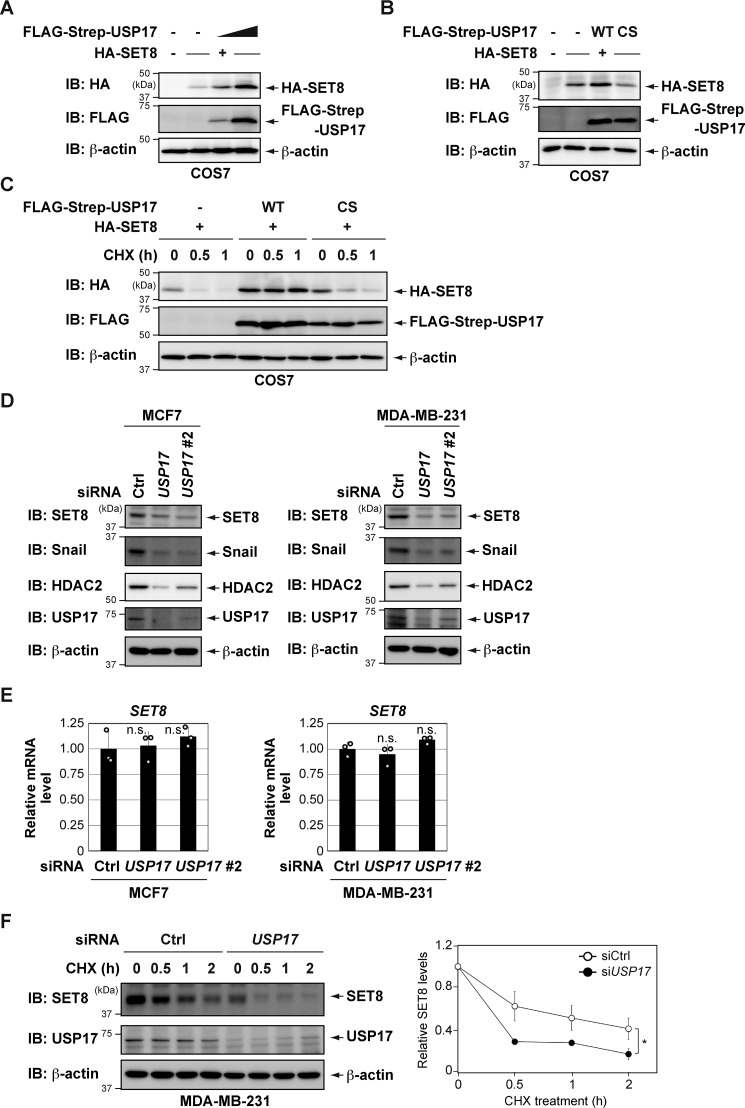
Figure 2.
USP17 regulates the stability of SET8 protein. A, HA-SET8 was coexpressed with increasing amounts of FLAG-Strep-USP17 in COS7 cells. After 24 h, cell lysates were immunoblotted (IB) with the indicated antibodies. B, HA-SET8 was coexpressed with FLAG-Strep-USP17 (either the WT or the catalytically inactive mutant (C89S)) in COS7 cells. After 24 h, cell lysates were immunoblotted with the indicated antibodies. CS, C89S. C, USP17 (WT), but not USP17 (C89S), delayed SET8 protein turnover. COS7 cells were transiently transfected with the indicated constructs. After 24 h, cells were treated with 25 μg/ml CHX for the indicated periods and harvested for immunoblotting with the indicated antibodies. D, depletion of USP17 decreases SET8 protein levels. Cells were transiently transfected with the indicated siRNAs. After 72 h, cell lysates were immunoblotted with the indicated antibodies. Ctrl, control. E, cells were transiently transfected with the indicated siRNAs for 72 h. The expression of each gene was assessed by qPCR. The expression level of SET8 was normalized to that of β-actin mRNA. Results are shown as mean ± S.D. (n = 3). F, time course of CHX treatment of MDA-MB-231 cells transiently transfected with the indicated siRNAs. The protein stability of SET8 was analyzed at the indicated times by immunoblot analysis (left panel). Quantification of the SET8 protein level was normalized to β-actin. Data represent the means and S.D. of three independent experiments (right panel). *, p < 0.05; n.s., not significant.