Abstract
Purpose
Although the enterococcal bloodstream infections (EBSI) are often observed in clinic, the mixed-EBSI are few reported. The aim of this study was to investigate the clinical characteristics and risk factors of mixed-EBSI in comparison with monomicrobial EBSI (mono-EBSI).
Methods
A single-center retrospective observational study was performed between Jan 1, 2013 and Dec 31, 2018 in a tertiary hospital. All patients with EBSI were enrolled, and their data were collected by reviewing electronic medical records.
Results
A total of 451 patients with EBSI were enrolled including 157 cases (34.8%) with mixed-EBSI. The most common co-pathogens were Coagulase-negative Staphylococcus (26.86%), followed by Acinetobacter baumannii (23.43%) and Klebsiella pneumoniae (8.57%). In multivariable analysis, burn injury (adjusted odds ratio [aOR], 7.39; 95% confidence interval [CI], 2.69–20.28), and length of prior hospital stay (aOR, 1.01; 95% CI, 1.00–1.02) were associated with mixed-EBSI. Patients with mixed-EBSI developed with more proportion of septic shock (19% vs. 31.8%, p=0.002), prolonged length of intensive care unit (ICU) stay [9(0,25) vs. 15(2.5,36), p<0.001] and hospital stay [29(16,49) vs. 33(18.5,63), p=0.031]. The mortality was not significantly different between mixed-EBSI and mono-EBSI (p=0.219).
Conclusion
A high rate of mixed-EBSI is among EBSI, and Acinetobacter baumannii is the second predominant co-existed species, except for Coagulase-negative Staphylococcus. Burn injury and length of prior hospital stay are independent risk factors for mixed-EBSI. Although the mortality is not different, patients with mixed-EBSI might have poor outcomes in comparison with mono-EBSI, which merits more attention by physicians in the future.
Keywords: bloodstream infections, mixed-enterococcal bloodstream infections, monomicrobial enterococcal bloodstream infections, clinical characteristics, risk factors
Introduction
Due to potentially serious consequences, bloodstream infections (BSI) are a growing worldwide concern.1 Enterococci is an important pathogen of BSI, which ranks the second leading cause of central line-associated bloodstream infection (16%) after Coagulase-negative Staphylococcus (CNS) (34.1%) according to the National Healthcare Safety Network’s report.2,3 The Enterococci becomes a significant pathogen, resulting from its ubiquitous distribution in the intestinal flora, the widespread uses of antibiotics and immunosuppressants, and the increase of invasive medical examinations and treatments in recent years.4,5 Enterococcal bloodstream infections (EBSI) are associated with significant morbidity (9%) and mortality (20–50%).6–9 In a recent Chinese report, Enterococcus accounted for 20% bloodstream infections with a mortality rate of 24%.10 Thus, EBSI is becoming a serious threat to public health with its rising prevalence, high morbidity and mortality, and huge care cost.11
Most of BSI are monomicrobial, but the trend of polymicrobial BSI is rising which accounted for 6–34% of BSI in previous studies.12–14 Polymicrobial BSI is generally associated with a higher acute physiology and chronic health evaluation II (APACHE II) scores, prolonged ICU and hospital stay, and a more severe prognosis than monomicrobial BSI in adults.12,14–17 In these previous studies,12,14–17 some limitations are existed as follows: (1) The clinical significance and outcomes of polymicrobial versus monomicrobial BSI were in indeed investigated, but few reports focused on a specific pathogen. Thus, the specific clinical features and outcomes between mixed-EBSI and mono-EBSI are still largely unknown. (2) The outcomes like 28-day mortality were poor in patients with polymicrobial BSI than monomicrobial BSI,14,16 while other studies showed that mixed-EBSI were not independently associated with mortality.18 Thus, the clinical outcomes between polymicrobial BSI and monomicrobial BSI are still controversial. (3) Some risk factors like recent chemotherapy/radiation and recent antibiotic exposure were observed for mixed-EBSI,18 but the main subjects were African Americans and Caucasians. In addition, the sample size in the study was relatively small (284 episodes). Herein, we performed the study for better understanding of the clinical characteristics and risk factors of mixed-EBSI in Chinese population.
Materials And Methods
Patients And Study Design
This single-center retrospective cohort study was conducted from January 2013 to December 2018 in the Second Affiliated Hospital, Zhejiang University School of Medicine, a 3200-bed tertiary health-care facility in Hangzhou, China. The present study received human research ethics approval (No. 2019–194) from the Ethics Committee of the Second Affiliated Hospital, Zhejiang University School of Medicine, and made sure that the personal data should be kept confidential. Due to the retrospective nature of the study, the Ethics Committee determined that patient consent was not required. In addition, a statement of permission from patients for submission the present study was not required as the study did not include any personal information.
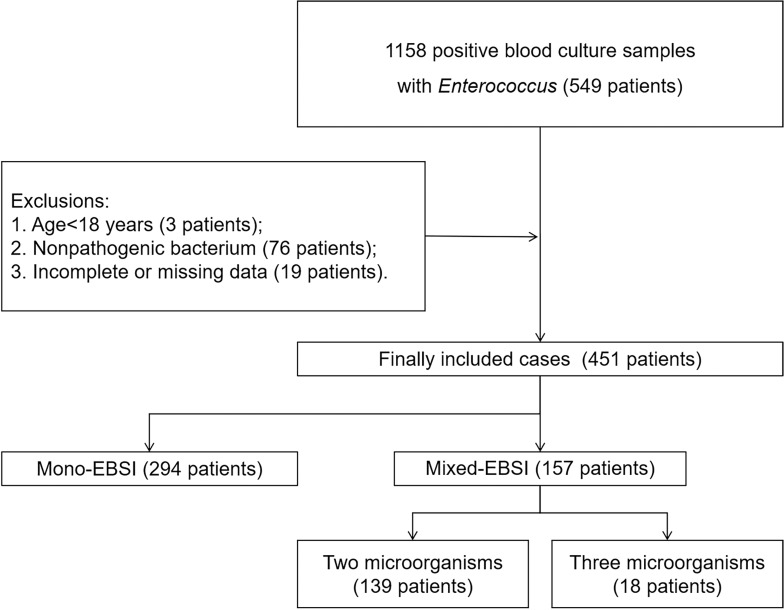
If any microorganisms other than Enterococcus were found in the same blood culture, the cases were retained. If only Enterococcus was found in multiple blood cultures of the same patient, the patients were only included once at the time of the first BSI with Enterococci. Exclusion criteria were as follows: a) Age<18 years old; b) Cases data were incomplete or missed; c) Enterococcus was considered as nonpathogenic bacterium. Common skin contaminant organisms (e.g., Bacillus spp., Corynebacterium spp., Micrococcus spp., Streptococci, Lactobacillus spp. and CNS) were considered as pathogens only when they were present in two or more consecutive blood cultures from separate blood draws. Thus, a total of 1158 blood culture specimens containing enterococcus were initially included, and final 451 cases were recruited with 157 cases for mixed-EBSI and 294 cases for mono-EBSI (Figure 1).
Figure 1.
Flowchart of study participant enrollment.
Abbreviation: EBSI, enterococcal bloodstream infection.
Data Collection
The patients’ data were collected by reviewing electronic medical records. The demographic data like age and gender, the clinical data including underlying diseases, sequential organ failure assessment (SOFA) score, Pitt bacteremia score, the Charlson Comorbidity Index (CCI) score, the APACHE II score in the first 24 h following the onset of BSI, the hospitalization wards, nosocomial infection or not, previous exposures (length of prior hospital stay, previous treatment such as surgical procedures, immunosuppressive agents, chemotherapeutic agents, radiation therapy, hyperalimentation, mechanical ventilation, renal replacement therapy, blood transfusion), and outcomes (length of hospital stay, length of ICU stay, cause septic shock and 28-day mortality) were collected. The microbiological data like species of Enterococcus, likely source of BSI, and sensitivity to antibiotics were also recorded. If the source of a BSI could not be attributed to any known source, it was classified as a primary BSI.19
Species Identification And Antibiotic Sensitivity Test
Blood was cultured using a BacT/ALERT 3D system (Becton-Dickinson, Sparks, MD, USA) in the microbiology laboratory. Species identification was performed using Bruker Daltonics DataAnalysis. Antibiotic susceptibility testing was performed using the VITEK 2 (Card number: AST-GN16; AST-GP67) system or the Kirby–Bauer Disk Diffusion method (Oxoid, UK) according to the recommendations proposed by the Clinical and Laboratory Standards Institute (CLSI).
Definitions
Diagnosis of EBSI was based on CDC definition for Bloodstream Infection Event.19 Onset of BSI was defined as the date when the blood culture was collected. Mixed-EBSI were defined as at least one nonenterococcal bacterial species isolated from one single blood culture sample.18 Nosocomial BSI was defined as the first positive blood culture obtained ≥48 h after hospital admission and with no evidence of infection at admission.9,20 Nonpathogenic bacterium was considered as contaminants, defined as one single positive blood culture in the absence of clinical manifestations.21 Appropriate antibiotic therapy was defined as an antibiotic regimen to which the index enterococcal isolate and co-pathogen (when applicable) were susceptible in vitro based on Clinical and Laboratory Standards Institute guidelines. Delayed antibiotic therapy was defined as therapy given more than 48 hrs after release of antibiotic susceptibility results.22 Sepsis and Septic shock were defined according to the new definition of Sepsis-3.23
Statistical Analysis
Statistical analysis was performed with SPSS 20.0 (IBM Corp, Armonk, NY, USA) software. Continuous variables were presented as mean ± standard deviation if normally distributed, and as median and interquartile range (IQRs) if nonnormally distributed. Continuous variables were compared by Student t test or Mann–Whitney U-test and enumeration variables were compared by Pearson χ2 or Fisher exact test, where appropriate. Variables that had significance at a p<0.05 level in the univariate analysis were considered candidates for the building of stepwise logistic regression multivariable models. A two-tailed p<0.05 was considered statistically significant.
Results
Demographic Characteristics
The demographic characteristics of these patients are summarized in Table 1. The median age was 63 years (IQR, 50,72), and 71% (320/451) of them were male. Solid tumor was the most common comorbidity (23.3%), followed by trauma (19.5%) and diabetes mellitus (16.2%). The most ward of EBSI occurrence was ICU (61.6%), followed by surgical ward (29%) and medical ward (9.1%). There was no significant difference in age or gender between groups of mixed-EBSI and mono-EBSI. In terms of co-morbidities, a significant high percentage of trauma or burn injuries was observed in mixed-EBSI compared with mono-EBSI (both p<0.05). In comparison with mono-EBSI, patients with mixed-EBSI presented a more severe condition, evidenced by a higher APACHE II score (median, 18 vs. 15, p=0.001), a higher SOFA score (median, 6 vs. 5, p=0.005) and a higher Pitt Bacteremia Score (median, 4 vs. 3, p<0.001), and displayed more need of ICU admission (56.5% vs. 71%, p=0.002) or invasive mechanical ventilation (63.3% vs. 78.3%, p=0.001). Although patient with mixed-EBSI was negatively correlated with admission to surgical wards (21% vs. 33.7%, p=0.005), which was not related to surgery (52.9% vs. 47.3%, p=0.258) and the use of parenteral nutrition (55.3% vs. 45.9%, p=0.125). Blood transfusion was significantly often in patients with mixed-EBSI than those with mono-EBSI (15.9% vs. 8.2%, p=0.012). A significant increase in central line indwelling was observed in mixed-EBSI compared with mono-EBSI (50.3% vs. 39.8%, p=0.032), but not for indwelling of urinary catheter or intraperitoneal drainage tube (both p>0.05). In addition, a longer hospital stay before onset of BSI was often seen in patients with mixed-EBSI than mono-EBSI (median, 12 vs. 8.5, p=0.001).
Table 1.
Demographic And Clinical Characteristics Of The Patients With Mono-EBSI Or Mixed-EBSI
| Characteristics | Total (n=451) | Mono-EBSI (n =294) | Mixed-EBSI (n =157) | P-value |
|---|---|---|---|---|
| Age, median years (IQR) | 63.0(50.0,72.0) | 63.0(51.0,73.0) | 61.0(47.0,71.0) | 0.317 |
| Male sex | 320(71.0%) | 212(72.1%) | 108(68.8%) | 0.460 |
| Co-morbidities | ||||
| Diabetes mellitus | 73(16.2%) | 44(15.0%) | 29(18.0%) | 0.336 |
| Chronic kidney disease | 29(6.4%) | 20(6.8%) | 9(5.7%) | 0.659 |
| Chronic liver disease | 17(3.8%) | 11(3.7%) | 6(3.8%) | 0.966 |
| COPD or Severe asthma | 27(6.0%) | 19(6.5%) | 8(5.1%) | 0.560 |
| Chronic cardiac insufficiency | 38(8.4%) | 26(8.8%) | 12(7.6%) | 0.662 |
| Solid tumour | 105(23.3%) | 75(25.0%) | 30(19.1%) | 0.125 |
| Trauma | 88(19.5%) | 45(15.3%) | 43(27.4%) | 0.002 |
| Burn injury | 29(6.4%) | 7(2.4%) | 22(14.0%) | <0.001 |
| Cerebrovascular accident | 69(15.3%) | 45(15.3%) | 24(15.3%) | 0.996 |
| CCI, median (IQR) | 3(2,5) | 4(2,5) | 3(1,5) | 0.089 |
| APACHE II score, median (IQR) | 16(11.21) | 15(10.20) | 18(13.22) | 0.001 |
| SOFA score, median (IQR) | 5(4,9) | 5(3,8) | 6(4,9) | 0.005 |
| Pitt Bacteremia Score, median (IQR) | 4(2,6) | 3(1,5) | 4(3,6) | <0.001 |
| Hospitalization ward | ||||
| Medical | 41(9.1%) | 29(9.9%) | 12(7.6%) | 0.435 |
| Surgical | 132(29.0%) | 99(33.7%) | 33(21.0%) | 0.005 |
| ICU | 278(61.6%) | 166(56.5%) | 112(71.0%) | 0.002 |
| Previous treatment | ||||
| Hyperalimentation | 219(48.6%) | 135(45.9%) | 84(55.3%) | 0.125 |
| Mechanical ventilation | 309(68.5%) | 186(63.3%) | 123(78.3%) | 0.001 |
| Antibiotic exposure | 426(94.5%) | 279(94.9%) | 147(93.6%) | 0.575 |
| Surgery | 222(49.2%) | 139(47.3%) | 83(52.9%) | 0.258 |
| Chemotherapy/radiation | 11(2.4%) | 6(2.0%) | 5(3.2%) | 0.453 |
| Renal replacement therapy | 38(8.4%) | 25(8.5%) | 13(8.3%) | 0.935 |
| Blood transfusion | 49(10.9%) | 24(8.2%) | 25(15.9%) | 0.012 |
| Invasive devices | ||||
| Central line | 196(43.5%) | 117(39.8%) | 79(50.3%) | 0.032 |
| Indwelling urinary catheter | 321(71.2%) | 203(69.0%) | 118(75.2%) | 0.172 |
| Intraperitoneal drainage | 87(19.3%) | 58(19.7%) | 29(18.5%) | 0.747 |
| Prior hospital stay, median days (IQR) | 9.0(4.0.19.0) | 8.5(3.0.15.3) | 12.0(5.0.21.0) | 0.001 |
| Nosocomial infection | 396(87.8%) | 253(86.1%) | 143(91.1%) | 0.120 |
Notes: Bold indicates P<0.05.
Abbreviations: COPD, chronic obstructive pulmonary disorder; CCI, Charlson Comorbidity Index; ICU, intensive care unit; APACHE, acute physiology and chronic health evaluation; SOFA, sequential organ failure assessment; IQR, interquartile range; EBSI, enterococcal bloodstream infections.
Biological Indicators
A comparison of biological indicators between mixed-EBSI and mono-EBSI is shown in Table 2. Procalcitonin (PCT) was higher in patients with mixed-EBSI than that with mono-EBSI (median, 0.405 vs. 0.76, p=0.003), whereas there were no significant differences in blood routine test, liver & kidney function.
Table 2.
Comparison Of Biological Indicators Between Groups Of Mixed-EBSI And Mono-EBSI
| Biological Indicators | Total (n=451) | Mono-EBSI (n =294) | Mixed-EBSI (n =157) | P-value |
|---|---|---|---|---|
| Temperature (°C) (IQR) | 39.0(38.4,39.3) | 39(38.3,39.3) | 39(38.5,39.5) | 0.057 |
| Blood routine test | ||||
| WBC (×109/L) (IQR) | 10.0(6.8,13.9) | 10.0(7.1,14.0) | 9.3(6.3,13.9) | 0.215 |
| Hematocrit (%) (IQR) | 26.5(22.3,31.9) | 27.1(22.3,32.4) | 25.0(22.3,30.8) | 0.068 |
| Platelet (×109/L) (IQR) | 156.0(101.1,246.0) | 158.0(103.0,237.3) | 155.0(98.0,254.5) | 0.870 |
| ANC (IQR) | 8.46(5.51,12.33) | 8.72(5.86,12.35) | 7.59(5.15,12.25) | 0.169 |
| Liver and kidney function | ||||
| Albumin (g/L) (mean±S.D.) | 31.07±5.99 | 31.09±5.92 | 31.03±5.15 | 0.930 |
| GPT (U/L) (IQR) | 37.0(20.0,60.0) | 34.5(19.0,63.3) | 41.0(21.0,71.5) | 0.227 |
| GOT (U/L) (IQR) | 37.0(26.0,75.0) | 36.0(24.8,72.0) | 39.0(18.8,81.0) | 0.150 |
| ALP (U/L) (IQR) | 105.0(72.0,150.0) | 108.0(71.8,153.5) | 99.0(72.0,142.0) | 0.394 |
| γ-GT (U/L) (IQR) | 47.0(25.0,105.0) | 49.5(24.0,101.0) | 47.0(27.0,113.0) | 0.526 |
| LDH (U/L) (IQR) | 290.0(211.0,401.0) | 283.0(210.8,385.3) | 301.0(215.0,460.5) | 0.267 |
| TBil (μmol/L) (IQR) | 16.5(10.5,30.5) | 15.7(10.0,27.6) | 17.8(11.9,33.3) | 0.162 |
| SCr (μmol/L) (IQR) | 62.0(44.0,89.0) | 62.5(44.0,88.0) | 60.0(43.5,92.0) | 0.725 |
| PCT (ng/mL) (IQR) | 0.53(0.20,2.00) | 0.405(0.17,1.52) | 0.76(0.26,3.52) | 0.003 |
Notes: Bold indicates P<0.05.
Abbreviations: WBC, white blood count; ANC, absolute neutrophil count; GPT, glutamic-pyruvic transaminase; GOT, glutamic-oxaloacetic transaminase; ALP, alkaline phosphatase; γ-GT, gamma glutamyl transpeptidase; LDH, lactic dehydrogenase; TBil, total bilirubin; SCr, serum creatinine; PCT, procalcitonin; IQR, interquartile range; EBSI, enterococcal bloodstream infections.
Independent Risk Factors For Mixed-EBSI
As shown in Table 3, multivariate logistic regression model analysis showed that the independent risk factors of mixed-EBSI were burn injury (adjusted odds ratio [aOR], 7.39; 95% confidence interval [CI], 2.69–20.28), and the days of prior hospital stay before onset of BSI (aOR, 1.01; 95% CI, 1.00–1.02).
Table 3.
Multivariable Logistic Regression Of Factors Associated With Mixed-EBSI
| Variable | Unadjusted OR (95% CI) | p-Value | Adjusted OR (95% CI) | p-Value |
|---|---|---|---|---|
| Trauma | 2.09(1.30,3.35) | 0.002 | 1.10(0.62,1.96) | 0.742 |
| Burn injury | 6.68(2.79,16.02) | <0.001 | 7.39(2.69,20.28) | <0.001 |
| APACHE II score | 1.04(1.02,1.07) | 0.003 | 1.03(0.98,1.09) | 0.289 |
| SOFA score | 1.05(1.01,1.11) | 0.032 | 0.97(0.89,1.06) | 0.556 |
| Pitt Bacteremia Score | 1.15(1.07,1.24) | <0.001 | 1.09(0.95,1.26) | 0.231 |
| ICU stay | 1.95(1.29,2.97) | 0.002 | 0.98(0.42,2.30) | 0.960 |
| Surgical | 0.52(0.33,0.83) | 0.005 | 0.76(0.34,1.70) | 0.499 |
| Prior Blood transfusion | 2.13(1.17,3.87) | 0.013 | 1.26(0.63,2.53) | 0.523 |
| Central line | 1.53(1.04,2.26) | 0.032 | 0.89(0.55,2.342) | 0.610 |
| Mechanical ventilation | 2.10(1.34,3.29) | 0.001 | 1.13(0.54,2.34) | 0.751 |
| Prior hospital stay | 1.01(1.00,1.02) | 0.006 | 1.01(1.00,1.02) | 0.026 |
Notes: Bold indicates P<0.05.
Abbreviations: ICU, intensive care unit; APACHE, acute physiology and chronic health evaluation; SOFA, sequential organ failure assessment; EBSI, enterococcal bloodstream infections.
Species Distribution Of Enterococcal Bloodstream Infections
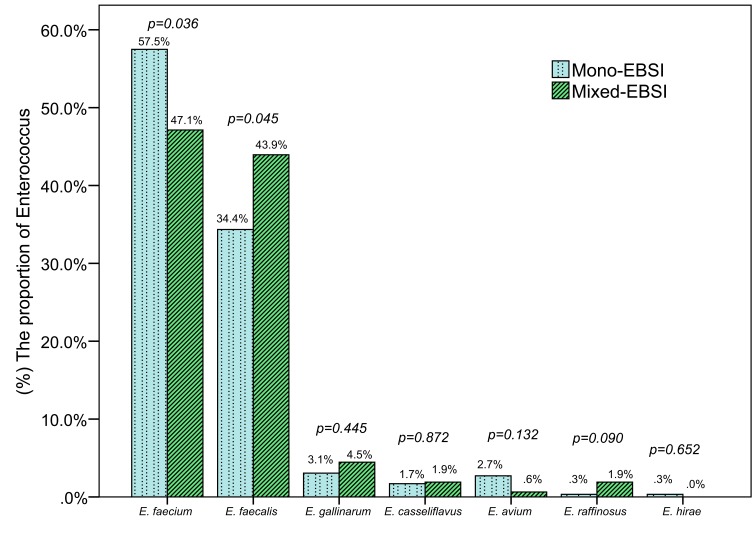
The most common Enterococcus species was Enterococcus faecium (E. faecium), which comprised 53.88% (243/451) of all episodes, followed by Enterococcus faecalis (E. faecalis) (37.69%, 170/451) and Enterococcus gallinarum (3.55%, 16/451) (Supplemental Figure 1). Of the 451 episodes of bacteremia, 294 (65.2%) were mono-EBSI and 157 (34.8%) were mixed-EBSI. The distribution comparison of Enterococcus species isolated from mixed-EBSI and mono-EBSI is shown in Figure 2, which showed the proportion of E. faecium or E. faecalis was significantly lower or higher in mixed-EBSI than that in mono-EBSI (47.1% vs. 57.5%, p=0.036; or 43.9% vs. 34.4%, p=0.045, respectively). A total of 175 other microorganisms in mixed-EBSI cases were isolated in 157 mixed-EBSI cases, with two microorganisms accounting for 88.5% (139/157) and three microorganisms for 11.5% (18/157). The most common co-pathogen was Gram-negative bacteria (57.1%), followed by Gram-positive bacteria (38.3%) and fungi (4.6%). In terms of the exacted microorganism, the most frequent pathogen was CNS (26.86%), followed by Acinetobacter baumannii (A. baumannii) (23.43%), Klebsiella pneumoniae (8.57%) and Staphylococcus aureus (S. aureus) (8%). The detailed distribution of additional organisms in mixed-EBSI is shown in Supplemental Figure 2.
Figure 2.
The distribution comparison of enterococcus species isolated from mixed-EBSI and mono-EBSI.
Abbreviation: EBSI, enterococcal bloodstream infection.
The source of EBSI was mainly from intra-abdominal (34.4%, 155/451), followed by primary BSI (28.8%, 130/451) and pneumonia (13.7%, 62/451). Compared with mono-EBSI, the sources of mixed-EBSI were more often from central venous catheter (12.7% vs. 6.1%, p=0.016) and the skin/soft tissue (16.6% vs. 5.8%, p<0.001), but less from abdominal cavity (26.1% vs. 38.8%, p=0.007) (Table 4).
Table 4.
Comparison Of Microbiological Characteristics In Patients With Mono-EBSI Or Mixed-EBSI
| Total (n=451) | Mono-EBSI (n =294) | Mixed-EBSI (n =157) | P-value | |
|---|---|---|---|---|
| Source of BSIs | ||||
| Intra-abdominal | 155(34.4%) | 114(38.8%) | 41(26.1%) | 0.007 |
| Primary BSI | 130(28.8%) | 84(28.6%) | 46(29.3%) | 0.871 |
| Pneumonia | 62(13.7%) | 41(13.9%) | 21(13.4%) | 0.867 |
| Skin and Soft tissue infection | 43(9.5%) | 17(5.8%) | 26(16.6%) | <0.001 |
| Central venous catheter | 38(8.4%) | 18(6.1%) | 20(12.7%) | 0.016 |
| Urinary tract infection | 12(2.7%) | 9(3.1%) | 3(1.9%) | 0.470 |
| Intracranial | 5(1.1%) | 5(1.7%) | 0(0.0%) | 0.168 |
| Endocarditis | 4(0.9%) | 4(1.4%) | 0(0.0%) | 0.303 |
| Othersa | 2(0.4%) | 2(0.7%) | 0(0.0%) | 0.545 |
| Antibiotic resistance of Enterococcusb | ||||
| Ampicillin (285 vs. 154)c | 229(52.2%) | 163(57.3%) | 66(42.9%) | 0.004 |
| Ciprofloxacin (294 vs. 157)c | 255(56.5%) | 172(58.5%) | 83(52.9%) | 0.250 |
| Tetracycline (208 vs. 112)c | 156(48.8%) | 93(44.7%) | 63(56.2%) | 0.049 |
| Erythromycin (236 vs. 113)c | 249(71.3.0%) | 172(72.9%) | 77(68.1%) | 0.359 |
| Levofloxacin (235 vs. 121)c | 210(59.0%) | 148(63.0%) | 62(51.2%) | 0.033 |
| Nitrofurantoin (239 vs. 132)c | 115(31.8%) | 84(35.1%) | 31(25.2%) | 0.054 |
| Teicoplanin (57 vs. 43)c | 1(1.0%) | 1(1.8%) | 0(0.0%) | 1 |
| Linezolid (288 vs. 152)c | 71(16.1%) | 41(14.2%) | 30(19.7%) | 0.136 |
| Vancomycin (294 vs. 157)c | 10(2.2%) | 8(2.7%) | 2(1.3%) | 0.505 |
| Treatment after the onset of BSIs | ||||
| Delayed antibiotic therapy | 74(16.4%) | 45(15.3%) | 29(18.5%) | 0.387 |
Notes: Bold indicates P<0.05; aSubmandibular gland, joint; bNot all agents listed tested in all isolates; cthe numbers in parentheses represent the total numbers of Enterococcus performed susceptibility test.
Abbreviation: EBSI, enterococcal bloodstream infections.
Antibiotic Resistance And Appropriate Therapy
The resistance of Enterococcus to vancomycin and teicoplanin in both groups of mixed-EBSI and mono-EBSI was very low (less than 3%) (Table 4). In comparison with mono-EBSI, the ratio of resistance of Enterococcus to tetracycline was significantly higher in mixed-EBSI groups (44.7% vs. 56.2%, p<0.05), but it was lower to ampicillin (42.9% vs. 57.3%) or levofloxacin (51.2% vs. 63.0%) (both, p<0.05). A total of 16.4% (74/451) patients did not receive appropriate therapy within 48 hrs after the release of antibiotic susceptibility results, but there was no difference between the two groups (15.3% vs. 18.5%, p=0.387) (Table 4).
Outcomes
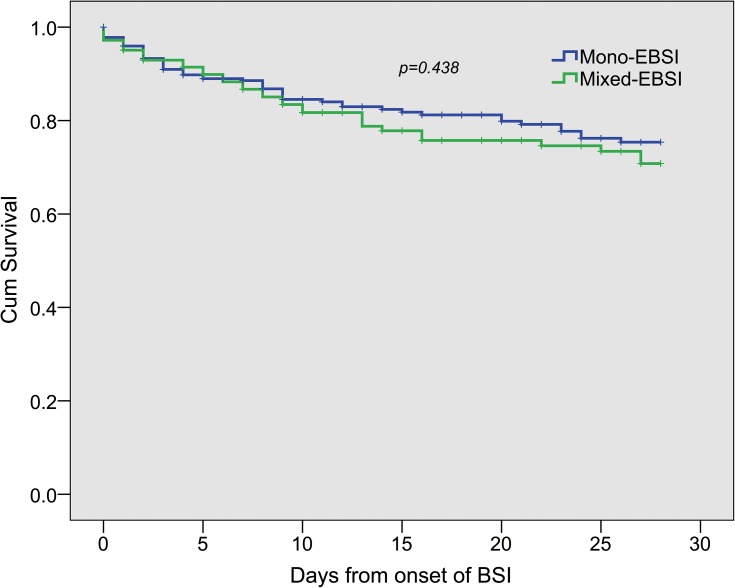
The comparison of prognosis between mixed-EBSI and mono-EBSI is shown in Table 5. The median length of hospital stay was 31 days (IQR, 16,53), and the median length of ICU stay was 11 days (IQR, 0,28). In comparison with mono-EBSI, patients with mixed-EBSI developed with more proportion of septic shock (19% vs. 31.8%, p=0.002), prolonged length of ICU stay [9(0,25) vs. 15(2.5,36), p<0.001] and hospital stay [29(16,49) vs. 33(18.5,63), p=0.031]. The 7-day, 14-day or 28-day mortality rates or in-hospital mortality in patients with mixed-EBSI were not different with those with mono-EBSI (Table 5, Figure 3).
Table 5.
Comparison Of Outcomes In Patients With Mono-EBSI Or Mixed-EBSI
| Outcomes | Total (n=451) | Mono-EBSI (n =294) | Mixed-EBSI (n =157) | P-value |
|---|---|---|---|---|
| Total Hospitalization days(M) (IQR) | 31.0(16.0,53.0) | 29.0(16.0,49.0) | 33.0(18.5,63.0) | 0.031 |
| Total ICU residence days(M)(IQR) | 11.0(0.0,28.0) | 9.0(0.0,25.0) | 15.0(2.5,36.0) | <0.001 |
| Septic shock (n,%) | 106(23.5%) | 56(19.0%) | 50(31.8%) | 0.002 |
| 7-day mortality (n,%) | 72(16.0%) | 44(15.0%) | 28(17.8) | 0.428 |
| 14-day mortality (n,%) | 95(21.1%) | 57(19.4%) | 38(24.2%) | 0.232 |
| 28-day mortality (n,%) | 111(24.6%) | 67(22.8%) | 44(28.0%) | 0.219 |
| In-hospital mortality (n,%) | 135(29.9%) | 80(27.2%) | 55(35.0%) | 0.084 |
Notes: Bold indicates P<0.05.
Abbreviations: M, median; IQR, interquartile range; ICU, intensive care unit; EBSI, enterococcal bloodstream infections.
Figure 3.
Kaplan-Meier estimates of survival in patients with mixed-enterococcal bloodstream infections and monomicrobial enterococcal bloodstream infections.
Abbreviation: EBSI, enterococcal bloodstream infection.
Discussion
In the current study, several important results were found. First, mixed-EBSI was no longer a rare event, and E. faecium (53.88%) was the most common pathogen. Second, some risk factors were found to be associated with mixed-EBSI, including ICU admission, a higher APACHE II score, a higher SOFA score, trauma, blood transfusion, mechanical ventilation and central venous catheter indwelling (Table 1). Moreover, burn injury and length of prior hospital stay were independent risk factors for mixed-EBSI. Third, although CNS had the highest proportion as co-pathogens in mixed-EBSI, Gram-negative bacteria remained the main co-pathogens in mixed-EBSI in comparison with Gram-positive bacteria. Last, patients with mixed-EBSI might have poor outcomes including higher occurrence of septic shock, prolonged lengths of ICU stay and hospital stay in comparison with mono-EBSI.
A high proportion (34.8%) of mixed-EBSI among EBSI was observed in the current study, which was consistent with other studies (28–44%).9,11,21,24 Previous studies14,25 showed that the rate of polymicrobial bacteremia was increasing over years, which might be explained by an increasing number of patients with central venous catheters and immunocompromised patients.25 In terms of the exact Enterococcus in the study, E. faecium (53.88%) was the most common pathogen, which was high than that in previous EBSI studies (less than 50%).5,9,21,26 A constant increase in the rate of E. faecium BSI was observed.4 In fact, the incidence of E. faecium BSI exceeding E. faecalis BSI was observed in a Swiss study and two Chinese studies.10,27,28 The exact reasons underlying the increased incidence of E. faecium infections are not yet well known, but might be related to increased resistance of E. faecium29 and enhanced virulence by acquiring new virulence factors.30
Like in previous studies,16,25,31–33 similar risk factors for mixed-EBSI in our study were found including ICU admission, a higher APACHE II score, a higher SOFA score, and a longer prior hospital stay before onset of BSI, burn injury or trauma, blood transfusion, mechanical ventilation and central venous catheter indwelling (Table 1). However, the CCI, reflecting the severity of underlying disease, did not show any difference in both groups (Table 1), which might be explained by the fact that CCI is inferior to APACHE II score to predict hospital mortality for ICU patients.34 Although recent chemotherapy/radiation and recent antibiotic exposure were positively associated with mixed-EBSI in a previous study,18 they were not independently associated with mixed-EBSI in our study. This might be due to a low proportion of patients (2.4%) receiving chemotherapy/radiation therapy in our study. Importantly, burn injury and length of prior hospital stay were independent factors for mixed-EBSI in the current study, which was consistent with a previous study showing that more than 12% of burn patients suffered from polymicrobial BSI.35 These results together suggest that burn patients are not only susceptible to BSI, but also to polymicrobial BSI including mixed-EBSI.
In our current study, the most common co-pathogen was CNS (26.86%), followed by A. baumannii (23.43%). It is worth noting that Gram-negative bacteria were still the main co-pathogen (57.1%) in comparison with Gram-positive bacteria (Supplemental Figure 2). Although CNS was the same most common co-pathogen, the second co-pathogen was A. baumannii in our study, whereas it was S. aureus in Lafnf’s study18 A high percentage of central venous catheter source of mixed-EBSI (38.7%) was observed in Lagnf’s study, while it only accounted for 12.7% in the current study. It is well known that the common pathogen of catheter-related bloodstream infections is Gram-positive bacteria especially S. aureus.36–38 Thus, this might partially explain a high proportion of S. aureus as a co-pathogen among polymicrobial EBSI in Lagnf’s study. In addition, we also found A. baumannii accounted for 38.8%, while S. aureus accounted for only 3.74% in post-neurosurgical intracranial infections in our previous study.39 This means gram-negative bacteria, especially A. baumannii, is the main pathogen in our hospital-acquired infection, as also observed in the distribution of co-pathogens in mixed-EBSI (57.1% for Gram-negative bacteria, while 38.3% for Gram-positive bacteria) in the current study. Taken together, A. baumannii was the second co-pathogen in mixed-EBSI, except for CNS.
Although a higher PCT value was observed in mixed-EBSI than that in mono-EBSI [0.76(0.26,3.52) vs 0.405(0.17,1.52), p=0.003] (Table 2), it may have no clinical meaning. It is worth noting that serum PCT level was often high in Gram-negative bacterium-induced BSI, whereas it was slightly increased or no effect after Gram-positive bacterium-mediated BSI.40–42 To this end, we stratified mixed-EBSI group into two sub-groups of mixed-EBSI with Gram-negative bacteria and mixed-EBSI with non-Gram-negative bacteria. Compared with mono-EBSI, PCT in mixed-EBSI with Gram-negative bacteria was significantly higher than that in mono-EBSI (median, 1.06 vs. 0.405, p<0.001), whereas it was similar to that in mixed-EBSI with non-Gram-negative bacteria (median, 0.380 vs. 0.405, p=0.582) (Supplemental Figure 3). These results suggest that we should keep in mind that mixed-EBSI including a Gram-negative bacterium might be present once EBSI is accompanied with a high serum PCT value.
Although patients with mixed-EBSI might have poor outcomes than those with mono-EBSI, the 28-day mortality was similar between the two groups (Table 5). This result was consistent with other studies showing that no correlation between polymicrobial EBSI and mortality was observed.10,11,21 Low percentage (less than 20%) of delayed antibiotic therapy, a high proportion (more than one third) of primary BSI as primary BSI has a lower mortality rate than secondary BSI,43 and a quite low proportion of vancomycin-resistant Enterococci (VRE) (Table 4), might be ascribed to the similar mortality observed in our study.
There were some limitations in this study. First, it was a retrospective study, and as a result, some important information or variable such as Glasgow coma scale score could not be obtained; In addition, it is hard to say cause and effect about the relationship of polymicrobial bacteremia and more serious condition, though patients with more severe illness and/or serious condition tend to get polymicrobial bacteremia. Second, although the data of this study were collected over a 6 years period in a tertiary hospital, it only represented a single center. In addition, the “primary BSI” described in the current study might have a bias, as the exact source of BSI was really hard to confirm by retrospective analysis. Thus, future multicenter prospective studies are needed to investigate the risk factors of mixed-EBSI.
Conclusion
Mixed-EBSI is not a rare event among total EBSI, and A. baumannii is the second predominant co-existed species, except for Coagulase-negative Staphylococcus. Many factors including trauma, burn injury, placement of central intravenous catheter, use of mechanical ventilation, need of blood transfusion, length of prior hospital stay, ICU admission, a higher APACHE II score, a higher SOFA score, and a higher Pitt Bacteremia score are associated with mixed-EBSI, whereas burn injury and length of prior hospital stay are independent risk factors. Although the mortality is not different, patients with mixed-EBSI might have poor outcomes, which merits more attention by physicians in the future.
Funding Statement
This work was supported in part by grants from the National Natural Science Foundation of China (No. 81570017, No. 81971871, GS Zhang; No. 81772110, ZC Zhang); and the Natural Science Foundation of Zhejiang Province (No. LY19H150007, GS Zhang).
Abbreviations
EBSI, enterococcal bloodstream infections; COPD, chronic obstructive pulmonary disorder; CCI, Charlson Comorbidity Index; WBC, white blood count; ANC, absolute neutrophil count; GPT, glutamic-pyruvic transaminase; GOT, glutamic-oxaloacetic transaminase; ALP, alkaline phosphatase; γ-GT, gamma glutamyl transpeptidase; LDH, lactic dehydrogenase; TBil, total bilirubin; SCr, serum creatinine; PCT, procalcitonin; mono-EBSI, monomicrobial enterococcal bloodstream infections; mixed-EBSI, mixed enterococcal bloodstream infections; OR, odds ratio; CI, confidence interval; ICU, intensive care unit; BSI, bloodstream infections; CNS, Coagulase-negative Staphylococcus; APACHE, acute physiology and chronic health evaluation; SOFA, sequential organ failure assessment; CDC, Centers for Disease Control and Prevention; IQR, interquartile range; CLSI, Clinical and Laboratory Standards Institute; E. faecium, Enterococcus faecium; E. faecalis, Enterococcus faecalis; A. baumannii, Acinetobacter baumannii; S. aureus, Staphylococcus aureus; VRE, vancomycin-resistant Enterococci; K. pneumoniae, Klebsiella pneumoniae; E. coli, Escherichia coli; P. aeruginosa, Pseudomonas aeruginosa; S. maltophilia, Stenotrophomonas maltophilia; S. viridans, Streptococcus viridans.
Ethical Approval
The present study received human research ethics approval from the Ethics Committee of the Second Affiliated Hospital, Zhejiang University School of Medicine. We make sure to keep patient data confidential and compliance with the Declaration of Helsinki.
Informed Consent
Due to the retrospective nature of the study, the Ethics Committee determined that no patient consent was required.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Rodriguez-Creixems M, Alcala L, Munoz P, Cercenado E, Vicente T, Bouza E. Bloodstream infections: evolution and trends in the microbiology workload, incidence, and etiology, 1985–2006. Medicine (Baltimore). 2008;87(4):234–249. [DOI] [PubMed] [Google Scholar]
- 2.Worth LJ, Spelman T, Bull AL, Brett JA, Richards MJ. Central line-associated bloodstream infections in Australian intensive care units: time-trends in infection rates, etiology, and antimicrobial resistance using a comprehensive Victorian surveillance program, 2009–2013. Am J Infect Control. 2015;43(8):848–852. doi: 10.1016/j.ajic.2015.03.036 [DOI] [PubMed] [Google Scholar]
- 3.Weiner LM, Webb AK, Limbago B, et al. Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the national healthcare safety network at the centers for disease control and prevention, 2011–2014. Infect Control Hosp Epidemiol. 2016;37(11):1288–1301. doi: 10.1017/ice.2016.174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Treitman AN, Yarnold PR, Warren J, Noskin GA. Emerging incidence of enterococcus faecium among hospital isolates (1993 to 2002). J Clin Microbiol. 2005;43(1):462–463. doi: 10.1128/JCM.43.1.462-463.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pinholt M, Ostergaard C, Arpi M, et al. Incidence, clinical characteristics and 30-day mortality of enterococcal bacteraemia in Denmark 2006–2009: a population-based cohort study. Clin Microbiol Infect. 2014;20(2):145–151. doi: 10.1111/1469-0691.12236 [DOI] [PubMed] [Google Scholar]
- 6.Noskin GA, Peterson LR, Warren JR. Enterococcus faecium and enterococcus faecalis bacteremia: acquisition and outcome. Clin Infect Dis. 1995;20(2):296–301. doi: 10.1093/clinids/20.2.296 [DOI] [PubMed] [Google Scholar]
- 7.Lodise TP, McKinnon PS, Tam VH, Rybak MJ. Clinical outcomes for patients with bacteremia caused by vancomycin-resistant enterococcus in a level 1 trauma center. Clin Infect Dis. 2002;34(7):922–929. doi: 10.1086/339211 [DOI] [PubMed] [Google Scholar]
- 8.DiazGranados CA, Zimmer SM, Klein M, Jernigan JA. Comparison of mortality associated with vancomycin-resistant and vancomycin-susceptible enterococcal bloodstream infections: a meta-analysis. Clin Infect Dis. 2005;41(3):327–333. doi: 10.1086/430909 [DOI] [PubMed] [Google Scholar]
- 9.Billington EO, Phang SH, Gregson DB, et al. Incidence, risk factors, and outcomes for Enterococcus spp. blood stream infections: a population-based study. Int J Infect Dis. 2014;26:76–82. doi: 10.1016/j.ijid.2014.02.012 [DOI] [PubMed] [Google Scholar]
- 10.Zhang Y, Du M, Chang Y, Chen LA, Zhang Q. Incidence, clinical characteristics, and outcomes of nosocomial Enterococcus spp. bloodstream infections in a tertiary-care hospital in Beijing, China: a four-year retrospective study. Antimicrob Resist Infect Control. 2017;6:73. doi: 10.1186/s13756-017-0231-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheah AL, Spelman T, Liew D, et al. Enterococcal bacteraemia: factors influencing mortality, length of stay and costs of hospitalization. Clin Microbiol Infect. 2013;19(4):E181–E189. doi: 10.1111/1469-0691.12132 [DOI] [PubMed] [Google Scholar]
- 12.Weinstein MP, Towns ML, Quartey SM, et al. The clinical significance of positive blood cultures in the 1990s: a prospective comprehensive evaluation of the microbiology, epidemiology, and outcome of bacteremia and fungemia in adults. Clin Infect Dis. 1997;24(4):584–602. doi: 10.1093/clind/24.4.584 [DOI] [PubMed] [Google Scholar]
- 13.Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis. 2004;39(3):309–317. doi: 10.1086/421946 [DOI] [PubMed] [Google Scholar]
- 14.Lin JN, Lai CH, Chen YH, et al. Characteristics and outcomes of polymicrobial bloodstream infections in the emergency department: A matched case-control study. Acad Emerg Med. 2010;17(10):1072–1079. doi: 10.1111/j.1553-2712.2010.00871.x [DOI] [PubMed] [Google Scholar]
- 15.Weinstein MP, Reller LB, Murphy JR. Clinical importance of polymicrobial bacteremia. Diagn Microbiol Infect Dis. 1986;5(3):185–196. doi: 10.1016/0732-8893(86)90001-5 [DOI] [PubMed] [Google Scholar]
- 16.Pavlaki M, Poulakou G, Drimousis P, et al. Polymicrobial bloodstream infections: epidemiology and impact on mortality. J Glob Antimicrob Resist. 2013;1(4):207–212. doi: 10.1016/j.jgar.2013.06.005 [DOI] [PubMed] [Google Scholar]
- 17.Sancho S, Artero A, Zaragoza R, Camarena JJ, Gonzalez R, Nogueira JM. Impact of nosocomial polymicrobial bloodstream infections on the outcome in critically ill patients. Eur J Clin Microbiol Infect Dis. 2012;31(8):1791–1796. doi: 10.1007/s10096-011-1503-8 [DOI] [PubMed] [Google Scholar]
- 18.Lagnf AM, Zasowski EJ, Claeys KC, Casapao AM, Rybak MJ. Comparison of clinical outcomes and risk factors in polymicrobial versus monomicrobial enterococcal bloodstream infections. Am J Infect Control. 2016;44(8):917–921. doi: 10.1016/j.ajic.2016.02.017 [DOI] [PubMed] [Google Scholar]
- 19.Bloodstream Infection (BSI) Events. Centers for Disease Control and Prevention. Available from: https://www.cdc.gov/nhsn/PDFs/pscManual/4PSC_CLABScurrent.pdf Accessed January2019.
- 20.Garner JS, Jarvis WR, Emori TG, Horan TC, Hughes JM. CDC definitions for nosocomial infections, 1988. American journal of infection control 1988;16(3):128–140. [DOI] [PubMed] [Google Scholar]
- 21.Suzuki H, Hase R, Otsuka Y, Hosokawa N. A 10-year profile of enterococcal bloodstream infections at a tertiary-care hospital in Japan. J Infect Chemother. 2017;23(6):390–393. doi: 10.1016/j.jiac.2017.03.009 [DOI] [PubMed] [Google Scholar]
- 22.Zasowski EJ, Claeys KC, Lagnf AM, Davis SL, Rybak MJ. Time is of the essence: the impact of delayed antibiotic therapy on patient outcomes in hospital-onset enterococcal bloodstream infections. Clin Infect Dis. 2016;62(10):1242–1250. doi: 10.1093/cid/ciw110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA. 2016;315(8):801–810. doi: 10.1001/jama.2016.0287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lautenbach E, Bilker WB, Brennan PJ. Enterococcal bacteremia: risk factors for vancomycin resistance and predictors of mortality. Infect Control Hosp Epidemiol. 1999;20(5):318–323. doi: 10.1086/501624 [DOI] [PubMed] [Google Scholar]
- 25.Bouza E, Burillo A, Munoz P, Guinea J, Marin M, Rodriguez-Creixems M. Mixed bloodstream infections involving bacteria and Candida spp. J Antimicrob Chemother. 2013;68(8):1881–1888. doi: 10.1093/jac/dkt099 [DOI] [PubMed] [Google Scholar]
- 26.Hamada Y, Magarifuchi H, Oho M, et al. Clinical features of enterococcal bacteremia due to ampicillin-susceptible and ampicillin-resistant enterococci: an eight-year retrospective comparison study. J Infect Chemother. 2015;21(7):527–530. doi: 10.1016/j.jiac.2015.04.001 [DOI] [PubMed] [Google Scholar]
- 27.Weisser M, Capaul S, Dangel M, et al. Additive effect of enterococcus faecium on enterococcal bloodstream infections: a 14-year study in a Swiss tertiary hospital. Infect Control Hosp Epidemiol. 2013;34(10):1109–1112. doi: 10.1086/673145 [DOI] [PubMed] [Google Scholar]
- 28.Zhao C, Chen H, Wang H, et al. [Analysis of pathogen spectrum and resistance of clinical common organisms causing bloodstream infections, hospital-acquired pneumonia and intra-abdominal infections from thirteen teaching hospitals in 2013]. Zhonghua Yi Xue Za Zhi. 2015;95(22):1739–1746. [PubMed] [Google Scholar]
- 29.Cattoir V, Giard JC. Antibiotic resistance in enterococcus faecium clinical isolates. Expert Rev Anti Infect Ther. 2014;12(2):239–248. doi: 10.1586/14787210.2014.870886 [DOI] [PubMed] [Google Scholar]
- 30.Nallapareddy SR, Singh KV, Murray BE. Contribution of the collagen adhesin Acm to pathogenesis of Enterococcus faecium in experimental endocarditis. Infect Immun. 2008;76(9):4120–4128. doi: 10.1128/IAI.00376-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pammi M, Zhong D, Johnson Y, Revell P, Versalovic J. Polymicrobial bloodstream infections in the neonatal intensive care unit are associated with increased mortality: a case-control study. BMC Infect Dis. 2014;14:390. doi: 10.1186/1471-2334-14-390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim SH, Yoon YK, Kim MJ, Sohn JW. Risk factors for and clinical implications of mixed Candida/bacterial bloodstream infections. Clin Microbiol Infect. 2013;19(1):62–68. doi: 10.1111/j.1469-0691.2012.03906.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reuben AG, Musher DM, Hamill RJ, Broucke I. Polymicrobial bacteremia: clinical and microbiologic patterns. Rev Infect Dis. 1989;11(2):161–183. doi: 10.1093/clinids/11.2.161 [DOI] [PubMed] [Google Scholar]
- 34.Quach S, Hennessy DA, Faris P, Fong A, Quan H, Doig C. A comparison between the APACHE II and Charlson Index Score for predicting hospital mortality in critically ill patients. BMC Health Serv Res. 2009;9:129. doi: 10.1186/1472-6963-9-129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zorgani A, Franka RA, Zaidi MM, Alshweref UM, Elgmati M. Trends in nosocomial bloodstream infections in a burn intensive care unit: an eight-year survey. Ann Burns Fire Disasters. 2010;23(2):88–94. [PMC free article] [PubMed] [Google Scholar]
- 36.Sato A, Nakamura I, Fujita H, et al. Peripheral venous catheter-related bloodstream infection is associated with severe complications and potential death: a retrospective observational study. BMC Infect Dis. 2017;17(1):434. doi: 10.1186/s12879-017-2757-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saliba P, Hornero A, Cuervo G, et al. Mortality risk factors among non-ICU patients with nosocomial vascular catheter-related bloodstream infections: a prospective cohort study. J Hosp Infect. 2018;99(1):48–54. doi: 10.1016/j.jhin.2017.11.002 [DOI] [PubMed] [Google Scholar]
- 38.Saliba P, Hornero A, Cuervo G, et al. Interventions to decrease short-term peripheral venous catheter-related bloodstream infections: impact on incidence and mortality. J Hosp Infect. 2018;100(3):e178-e186. doi: 10.1016/j.jhin.2018.06.010 [DOI] [PubMed] [Google Scholar]
- 39.Pan S, Huang X, Wang Y, et al. Efficacy of intravenous plus intrathecal/intracerebral ventricle injection of polymyxin B for post-neurosurgical intracranial infections due to MDR/XDR Acinectobacter baumannii: a retrospective cohort study. Antimicrob Resist Infect Control. 2018;7:8. doi: 10.1186/s13756-018-0305-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yu Y, Li XX, Jiang LX, et al. Procalcitonin levels in patients with positive blood culture, positive body fluid culture, sepsis, and severe sepsis: a cross-sectional study. Infect Dis (Lond). 2016;48(1):63–69. doi: 10.3109/23744235.2015.1082618 [DOI] [PubMed] [Google Scholar]
- 41.Charles PE, Ladoire S, Aho S, et al. Serum procalcitonin elevation in critically ill patients at the onset of bacteremia caused by either Gram negative or Gram positive bacteria. BMC Infect Dis. 2008;8:38. doi: 10.1186/1471-2334-8-38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leli C, Ferranti M, Moretti A, Al Dhahab ZS, Cenci E, Mencacci A. Procalcitonin levels in gram-positive, gram-negative, and fungal bloodstream infections. Dis Markers. 2015;2015:701480. doi: 10.1155/2015/105358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Renaud B, Brun-Buisson C. Outcomes of primary and catheter-related bacteremia. A cohort and case-control study in critically ill patients. Am J Respir Crit Care Med. 2001;163(7):1584–1590. doi: 10.1164/ajrccm.163.7.9912080 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Bloodstream Infection (BSI) Events. Centers for Disease Control and Prevention. Available from: https://www.cdc.gov/nhsn/PDFs/pscManual/4PSC_CLABScurrent.pdf Accessed January2019.