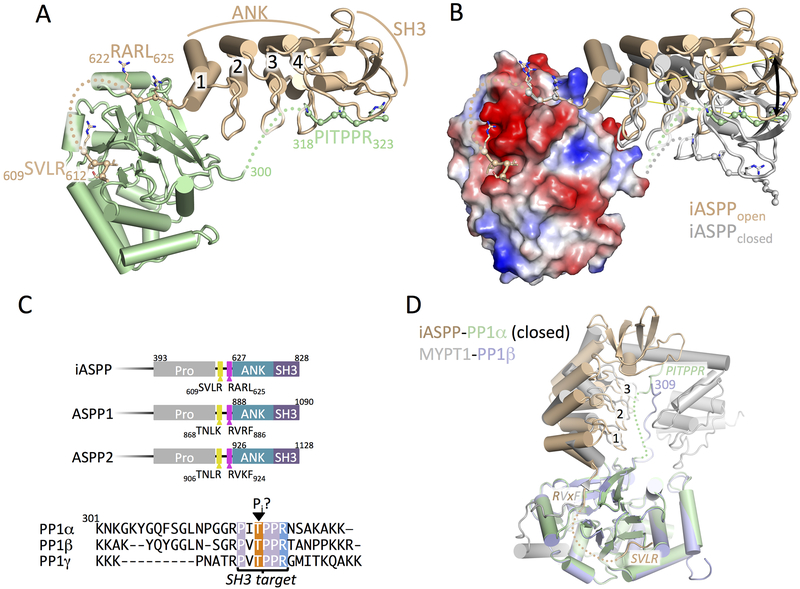
Figure 1.
Crystal structure of iASPP608–828 bound to PP-1cα.
(A) Overview of the structure of iASPP608–828 bound to PP-1cα. iASPP is brown, PP-1cα is in green. Peptide segments involved in key protein-protein contacts are illustrated with Cα spheres and the four ANK repeats are numbered. Regions of polypeptide chain not visible in the electron density are indicated by dotted lines.
(B) Comparison of the two iASPP608–828-PP-1cα complexes in the asymmetric unit, aligned on PP-1cα, with the second iASPP in the closed conformation colored grey and an electrostatic charge surface displayed for PP-1cα. The ~22° angular difference in the orientation of the two iASPPs relative to PP-1cα is indicated.
(C) Top panel, comparison of the primary structures of the C-terminal regions of iASPP, ASPP1 and ASPP2 with key domains and motifs indicated. Bottom panel, sequence alignment of the C-terminal unstructured tails of the three PP-1cα isoforms.
(D) Structural comparison of the closed complex of iASPP-PP-1cα with the structure of MYPT1-PP-1β, aligned on PP-1cα with the different chains colored as indicated in the figure. Crystallographic statistics are given in Table 1.