Abstract
Background
Acute respiratory infections (ARI) are the leading cause of death worldwide, especially among children. The majority of these infections in children are of viral etiology. In this study, we evaluated the incidence of viral ARI among children in Lebanon.
Patients and Methods
Children presenting with symptoms of ARI were prospectively recruited between September 2009 to February 2012. Nasopharyngeal aspirates were obtained from patients and screened for 11 respiratory viruses using a multiplex Luminex-based PCR assay.
Results
Two hundred twenty-one patients were recruited with a median age of 1 year (IQR: 0 – 5). Out of 221 patients, 116 (52.5%) were positive for at least one virus, the majority (103/116; 88.8%) of which were in children under 6-year of age. Overall, 188 viruses were detected. Rhinovirus (RhV) was the most common virus detected in 81 (69.8%) patients followed by coxsackie virus and echovirus (CVEV) which were detected as one target in the panel in 45 (38.8%), and parainfluenza viruses (PIV types: 1, 2, 3, 4) in 24 (20.7%) patients. Coinfection with more than one virus was detected in 49 (42.9%) patients. RhV and CVEV were the most common viruses associated with co-infections and higher risk of rhinorrhea.
Conclusions
Viral pathogens account for at least half of the ARIs in Lebanon, with a high frequency of co-infections being detected.
Keywords: Children Viral infections, Respiratory, Luminex, Molecular diagnosis
Introduction
Acute respiratory tract infections (ARIs) are among the most common reasons for primary care consultations.1 The World Health Organization (WHO) ranks ARIs as the fourth major killer after cardiovascular diseases, general infections and parasitic diseases, and cancer.2 ARIs cause 4 million death globally. The burden is especially high in children where ARIs are responsible for 11–22% of deaths.3 ARIs can lead to severe complications requiring hospitalizations and can have fatal outcomes.
Viruses are the most common etiology of ARIs in children.6,7 These include rhinovirus (RhV), respiratory syncytial virus (RSV), influenza (IFN), parainfluenza virus (PIV), coronavirus (CoV), human metapneumovirus (hMPV), enteroviruses (EV), adenovirus (AdV), and human bocavirus (HBoV).6,8,9 Each of these viruses poses a significant health burden. Nair et al. estimated that 111 500 deaths in children <5 years were attributable to influenza-associated lower respiratory tract infections (LRI) in 2008, the vast majority of which occurred in developing countries.10 RSV was estimated to have caused 33.8 million LRI episode in children under five, of which 3.4 million were severe causing up to 199 000 deaths.11 In addition to the health burden of viral respiratory tract infections (RTIs), the economic impact is also high if we account for health care costs (direct cost) and loss of productivity (indirect cost). A study by Fendrik et al. estimated the total economic impact of non-influenza-related viral RTIs in the United States at $40 billion annually.12
The advancements that have been achieved in developing antiviral drugs, some of which have already been approved, against respiratory viruses allow for targeted therapy of viral ARIs.13–15 This possibility calls for better and faster diagnosis of the etiologic agents in ARI patients to benefit from the full potential of these drugs.6
Furthermore, ARIs are associated with the greatest amount of excess use of antibiotics that has led to unprecedented increase in antimicrobial drug resistance;16,17 therefore, proper and timely diagnosis of viral infections can help reduce unnecessary antibiotic prescriptions.5,6
In Lebanon, studies investigating the viral etiologies of ARIs are very scarce. In this study, we determined the viral etiologies among ARI patients at a tertiary care hospital that serves an ethnically and socio-economically diverse patient population.
Materials and Methods
Patients and samples collection
Infants and children younger than 18 years of age with symptoms of ARI disease presenting to the emergency department or the departments of pediatrics of the American University of Beirut Medical Center (AUBMC), Beirut, Lebanon were prospectively recruited between September 2009 to February 2012. An ARI was defined as an acute infection of the upper and lower respiratory airways. Recruited patients had one or more of the following symptoms: fever, cough, sore throat, rhinorrhea, headache, conjunctivitis, wheezing, dyspnea, and vomiting.
Medical history and demographic data were obtained from the patients and their medical records. A respiratory sample was collected and stored at −80°C for viral assessment. The study was approved by the Institutional Review Board (IRB) of the AUBMC, and written informed consent was obtained from all parents.
Nucleic acid extraction and viral detection
Nucleic acid was extracted from clinical specimens by using the QIAamp MinElute Virus Spin kit (Qiagen) according to the manufacturer’s protocol. A 200 μl aliquot of each specimen was used for nucleic extraction. Specimens were then analyzed by the ResPlex II panel (Qiagen) using the manufacturer’s protocol.
The ResPlex II panel can detect 11 viral targets: RSVA, RSVB, INFA, INFB, PIV1, PIV2, PIV3, PIV4, hMPV, CVEV (coxsackievirus and echovirus), and RhV. Briefly, 10 μl of each specimen were added to 40 μl reverse transcription-PCR (ResPlex II) master mix, including the supplied primers. Targets were detected by mixing 5 μl portions of amplification products with ResPlex II bead in hybridization buffer at 52°C for 10 min. Streptavidin-phycoerythrin conjugate was added, and mixtures were incubated at 52°C for a further 5 min before the addition of stop buffer. The samples were then analyzed on a Luminex Bio-Rad BioPlex 200 System (Bio-Rad Laboratories) using Bio-Rad BioPlex Manager software. The cutoff value for each target was determined, as previously described by Lia et al.19
Statistical analysis
The data were checked for completeness, and responses were coded and entered into the Statistical Package for the Social Sciences (SPSS) software version 23 for Windows, which was later used for statistical analyses.32 Descriptive statistics were presented to summarize the study variables of interest as counts and percentages for the categorical variables and as medians and Interquartile Range (IQR) for the continuous ones. The Chi-square test was used to calculate the association between two categorical variables. Pearson’s chi-square analysis with Bonferroni-Holm p-value correction was used for multiple comparisons to assess infectivity enhancing correlations. Univariate and multivariate logistic regression analyses were applied to determine which factors are associated with rhinorrhea. In the regression model, rhinorrhea was used as the dependent variable. Odds ratios and their respective 95% confidence intervals were calculated. For all analysis done, a p-value of less than 0.05 was considered statistically significant.
Results
Patient characteristics
A total of 221 specimens were collected from children presenting with symptoms of ARI between September 2009 and February 2012 (Table 1). The socio-demographic characteristics of the study patients are presented in Table 1. Overall, the study consisted of 130 males (58.8%) and 91 females (41.2%) with patients’ median age of 1 (IQR: 0 – 5) years. Seventy-four patients (33.5%) were children under one year of age, 105 (47.5%) were between 1 to 6 years old, 33 (14.9%) were 6 to 12 years old, and 9 (4.1%) were 12 to 18 years old. Sixty-seven (30.3%) of the children had an underlying disease (asthma, immune-deficiency, allergic rhinitis, or cystic fibrosis). At the time of diagnosis 13 (5.8%) patients were receiving chemotherapy, and 95 (43%) had received an antibiotic.
Table 1.
Demographics of the ARI patients.
| Characteristics | Patients (n=221) |
|---|---|
| Median age in years (IQR boundaries) | 1 (0 – 5) |
| Age group: | |
| < 1 year | 74 (33.5%) |
| 1–6 years | 105 (47.5%) |
| 6–12 years | 33 (14.9%) |
| 12–18 years | 9 (4.1 %) |
| Gender: | |
| Male | 130 (58.8%) |
| Underlying conditions: | |
| Asthma | 34 (15.4%) |
| Immune-deficiency | 6 (2.7%) |
| Allergic Rhinitis | 23 (10.4%) |
| Cystic fibrosis | 4 (1.8%) |
| Chemotherapy | 13 (5.9%) |
| Prior antibiotics | 95 (43%) |
IQR: interquartile range.
Virological characterization
Samples were screened for 11 virus targets included in the ResPlexII respiratory panel. Of the 221 ARI episodes, 116 (52.5%) were confirmed to be of viral etiology being positive for at least one of the virus targets tests (Table 2). The majority (n=103; 88%) of viral ARI episodes were observed in children under 6-year of age (chi-square, p<0.05).
Table 2.
Epidemiologic and clinical characteristics of children with viral acute respiratory infection.
| Respiratory Viruses or Infection Status | ||||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Any viral etiology n=116 |
RSV n = 18 |
INF n = 13 |
PIV n = 24 |
hMPV n=3 |
CVEV n = 45 |
RHV n = 81 |
Coinfection (≥ 2 viruses) n=49 | |
| Age: | ||||||||
| < 1 year | 43 (37.1) | 7 (38.9) | 2 (15.4) | 8 (33.3) | 0 (0) | 19 (42.2) | 30 (37.0) | 18 (36.7) |
| 1–6 years | 60 (51.7) | 10 (55.6) | 8 (61.5) | 14 (58.3) | 3 (100) | 24 (53.3) | 41 (50.6) | 28 (57.1) |
| 6–12 years | 13 (11.7) | 1 (5.6) | 3 (23.1) | 2 (8.3) | 0 (0) | 2(4.4) | 10 (12.3) | 3 (6.1) |
| 12–18 years | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Gender: | ||||||||
| Female | 49 (42.2) | 6 (33.3) | 5 (38.5) | 6 (25) | 1 (33.3) | 20 (44.4) | 35 (43.2) | 20 (40.8) |
| Male | 67 (57.8) | 12 (66.7) | 8 (61.5) | 18 (75) | 2 (66.7) | 25 (55.6) | 46 (56.8) | 29(59.2) |
| Underlying conditions: | ||||||||
| Asthma | 18 (15.5) | 3 (16.7) | 4 (30.8) | 3 (12.5) | 1 (33.3) | 7 (15.6) | 12 (14.8) | 9 (18.4) |
| Immune-deficiency | 2 (1.7) | 0 (0) | 0 (0) | 1 (4.2) | 0 (0) | 0 (0) | 1 (1.2) | 0 (0) |
| Allergic Rhinitis | 11 (9.5) | 2 (11.1) | 3 (23.1) | 1 (4.2) | 0 (0) | 5 (11.1) | 5 (6.2) | 4 (8.2) |
| Cystic fibrosis | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Chemotherapy | 7 (6) | 0 (0) | 1 (7.7) | 1 (4.2) | 0 (0) | 2(4.4) | 5 (6.2) | 1 (2) |
| Prior antibiotics | 42 (36.2) | 5 (27.8) | 3 (23.1) | 13 (54.2) | 0 (0) | 20 (44.4) | 27 (33.3) | 15 (30.6) |
| Symptoms: | ||||||||
| Fever ≥ 38°C | 99 (85.3) | 16 (84.2) | 12 (92.3) | 19 (79.2) | 3 (100) | 38 (84.4) | 70 (86.4) | 43 (87.8) |
| Cough | 102 (87.9) | 18 (100) | 13 (100) | 22 (91.7) | 3 (100) | 39 (86.7) | 72 (88.9) | 46 (93.9) |
| Sore throat | 14 (12.1) | 3 (23.1) | 3 (23.1) | 4 (16.7) | 0 (0) | 4 (8.9) | 7 (8.6) | 6 (12.2) |
| Rhinorrhea | 95 (81.9) | 16 (88.9) | 13 (100)a | 19 (79.2) | 3 (100) | 41 (91.1)b | 65 (80.2)a | 45 (91.8)c |
| Headache | 10 (8.6) | 1 (5.6) | 3 (23.1) | 3 (12.5) | 1 (33.3) | 2 (4.4) | 8 (9.9) | 6 (12.2) |
| Conjunctivitis | 14 (12.1) | 1 (5.6) | 3 (23.1) | 6 (25) | 0 (0) | 5 (11.1) | 7 (8.6) | 7 (14.3) |
| Wheezing | 43 (37.1) | 10 (55.6) | 4 (30.8) | 9 (37.5) | 1 (33.3) | 21 (46.7) | 29 (35.8) | 20 (40.8) |
| Dyspnea | 53 (45.7) | 12 (66.7) | 7 (53.8) | 16 (66.7) | 1(33.0) | 24 (53.3) | 37 (45.7) | 27 (55.1) |
| Vomiting | 49 (42.2) | 8 (44.4) | 6 (46.2) | 12 (50.0) | 0 (0) | 25 (55.6) | 35 (42.0) | 26 (53.1) |
RSV: Respiratory syncytial virus, INF: Influenza virus, PIV: Parainfluenza virus, hMPV: human Metapneumovirus, CVEV: Coxsackie/Echovirus, RHV: Rhinovirus.
p value <0.05,
p value <0.01,
p value <0.001.
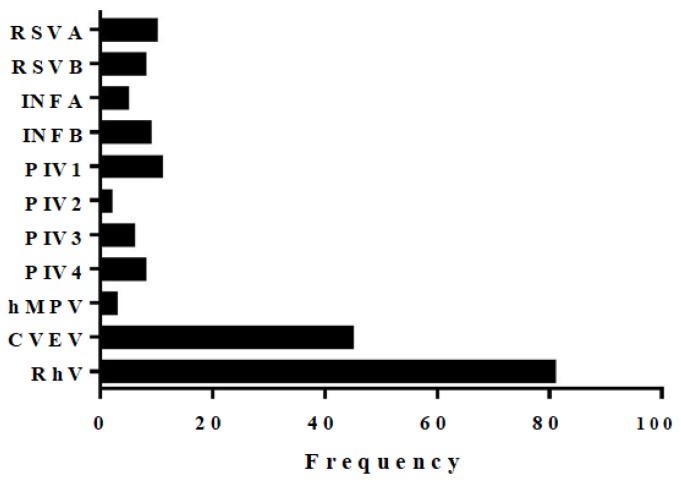
Figure 1 shows the frequency of each of the viruses detected in the study population. Overall 188 viruses were detected. Rhinovirus (RhV) was the most common virus detected in 81 (69.8%) patients followed by coxsackie virus and echovirus (CVEV) which were detected as one target in the panel in 45 (38.8%) patients, parainfluenza viruses (PIV types: 1, 2, 3, 4) in 24 (20.7%) and respiratory syncytial virus (RSV types A and B) in 18 (15.5%). Coinfection with more than one virus was detected in 49 (42.9%) patients (Figure 2). A significant majority (n=46; 93.9%; chi-square p-value< 0.05) of coinfections occurred in children under six years of age. The most frequent viral co-infections involved two viruses (n=33), 10 cases had a triple infection, 5 had four viruses detected, and one case had five viruses. Almost half cases of Rhinovirus (50.6%) were positive for at least another virus in the panel. CVEV positive cases also had a high rate of coinfection (73%). Moreover, hMPV and INFB were detected in 9 samples, and all were co-infected.
Figure 1.
Distribution of viruses among 221 patients with medically attended acute respiratory infections.
Figure 2.
Prevalence of virus co-detection among the study population.
Table 3 summarizes the correlation of different viruses among our patients. Several correlations enhancing infectivity were evident in our analysis. Of note, RhV was the most frequently detected virus in co-infections and was significantly associated with RSVB, INFB, PIV3, hMPV, and CVEV.
Table 3.
Cross-tabulation of the virus frequency among ARI patients.
| Virus | RSVA | RSVB | INFA | INFB | PIV1 | PIV2 | PIV3 | PIV4 | hMPV | CVEV | RhV |
|---|---|---|---|---|---|---|---|---|---|---|---|
| RSVA | 10 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 5* | 6 |
| RSVB | 8 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 2 | 6* | |
| INFA | 5 | 1 | 1 | 0 | 1* | 0 | 0 | 1 | 3 | ||
| INFB | 9 | 2* | 0 | 5** | 1 | 0 | 2 | 9** | |||
| PIV1 | 11 | 0 | 3** | 0 | 0 | 1 | 3 | ||||
| PIV2 | 2 | 0 | 0 | 0 | 1 | 1 | |||||
| PIV3 | 6 | 0 | 0 | 1 | 5* | ||||||
| PIV4 | 8 | 0 | 4* | 5 | |||||||
| hMPV | 3 | 0 | 3* | ||||||||
| CVEV | 45 | 28** | |||||||||
| RhV | 81 |
Correlation is significant at the 0.05 level (2-tailed).
Correlation is significant at the 0.01 level (2-tailed).
Underlying conditions and clinical presentation
We next analyzed the correlation between each of the viral etiologies or co-infection with the underlying conditions (Table 2). To simplify the analysis, we treated subtypes or genotypes of a virus as a single group (e.g. RSV for RSVA and RSVB, etc.). Having an underlying condition or receiving chemotherapy or a course of antibiotics were not found to be a risk factor for having a viral etiology or co-infection (Table 2). Additionally, we investigated the seasonal variation of viruses. Rhinovirus infections were detected throughout the year however the peak rate occurred during the main rainy months (November, December), likely for Coxsackie/Echovirus and RSV. On the other hand, Influenza A virus infections had a peak in the fall (September, October); (Figure 3). In terms of clinical symptoms, fever, cough and rhinorrhea were major symptoms observed in most of the patients infected with one or more respiratory virus (Table 2). Chi-square analysis revealed a significant correlation between rhinorrhea and INF, CVEV, and RhV and coinfection (Table 2). Bivariate logistic regression was then performed to determine the risk associated with these infections. Our analysis revealed that RhV or CVEV infected patients or patients infected with more than one virus were more likely to have rhinorrhea (OR for RhV: 2.25; CI: 1.18 – 4.31; OR for CVEV: 5.57; CI: 1.90 – 16.28; OR for co-infection: 6.34; CI: 2.17–18.44).
Figure 3.
Seasonal distribution of viruses. The figure describes the seasonal variation of respiratory viruses in the positive pediatric samples.
A multivariate logistic regression model was used to examine the correlates of rhinorrhea in the study patients. Variables were put in the model in order of strength of their correlation with rhinorrhea as per the bivariate analysis. The effect of each variable on the model was assessed, and the variable was kept if it significantly contributed to a better fit of the model. The final model included the following variables: RhV and CVEV. The results of the multivariate model showed that CVEV was independently associated with rhinorrhea (OR: 4.73; CI: 1.59 – 14.07). CVEV infected patients were 4.73 times more likely to have rhinorrhea compared to none-CVEV patients controlling for RhV. Unlike the bivariate analysis, RhV was not significantly associated with rhinorrhea (OR: 1.78; CI: 0.91 – 3.48). RhV infected patients were 1.78 times more likely to have rhinorrhea compared to none-RhV patients controlling for CVEV; however, this was not statistically significant.
Discussion
We demonstrated that viral infections are responsible for at least half of the ARIs in children in Lebanon. Rhinovirus infection was the most common etiology of ARI consistent with other studies from Lebanon and other countries.18,20,21 In neighboring Jordan and Egypt rhinovirus incidence was second to RSV, but the population captured in these studies was younger than that included in our study.22,23 The overall viral ARI incidence (52.5%) in our study lower than that recently reported by Finianos et al. (70%) in Lebanon.18 Both studies targeted children; however, Finianos et al. screened their specimens for more viral targets than those included in our analysis. In our study we did not test for HCoV, AdV, EV, and HBoV which collectively accounted for 50% of viral ARI in the study by Finianos et al.
The coinfection rate in our study (42.9%) was higher than that previously reported in Lebanon (37%), Qatar (21.4%), and Egypt (10.8%).18,23,24 This incongruence could be because CVEV, which was frequently detected with other viruses in our study, was not screened in the previous studies from the region.18 CVEV infections are not commonly reported in studies investigating respiratory infections. In our study, CVEV infection constituted 38.8% of all viral ARI cases and was independently associated with rhinorrhea. This incidence is much higher than that reported in other countries. A recent study in Latin America reported that CVEV was associated with 3% of the ARI cases.25 In Central America, CVEV was even much lower (0.3%).26 The very low prevalence of CVEV in other regions might have discouraged its testing. Given the high prevalence of CVEV in Lebanon, we recommend testing for enteroviruses, including (CVEV).
Co-infections were found to be more common younger children in Lebanon, and that is similar to a previous study done in Mexican children showing that the majority of coinfections occur in children <6 months of age.27 Younger children are likely to be more prone to infections due to their lack or still weak immunity to respiratory viruses. The effect of coinfections on disease outcomes is not well understood.28 Patients coinfected with pandemic H1N1 influenza and rhinovirus tended to have milder clinical severity when compared with non-rhinovirus coinfections;29 while the patients coinfected with HMPV and RSV were prone to a higher risk of severe bronchiolitis.30 Additionally, the prevalence and severity of obstructive airway disease were higher in patients with coinfections.31 In our study, coinfection was associated with higher risk of rhinorrhea but not with more severe symptoms like dyspnea. In contrast, some studies showed no correlation between coinfection status and clinical severity.32,33
The complexity of viral coinfections and the large number of respiratory viruses involved make challenging to study the effect of coinfection on disease outcome in a clinical setting. Therefore, there is a need for developing in vitro or in vivo models to allow a better understanding of coinfections. For example, dual infection with INF was shown to suppress RSV growth in vitro.34 The suppression of RSV by INF was suggested to be due to competition for protein synthesis and budding from the cell surface. Further studies are warranted to investigate the interactions among respiratory viruses during coinfection and their effect on the host.
Conclusions
Our study had a couple of limitations. First, we have not screened for HBoV, and HCoV which are not included in ResPlex II kit and thus the prevalence of viral ARI is expected to be higher than 52%. Another limitation was our inability to rule out bacterial etiologies which was not tested for in the current study. In conclusion, viral etiologies contribute to a large proportion of ARIs many of which involve more than one viral agent.
Figure 4.
A. Distribution of positive signals on 10-plex panels of ResPlex II assay. B. Frequencies of virus detected as a single or in combination with other viruses. Numbers in bars represent the absolute numbers of infection per virus. RSV, respiratory syncytial virus; INFA, Influenza type A virus; INFB, Influenza type B virus; hMPV, human metapneumovirus; CVEV, Coxsackie/Echovirus.
Footnotes
Competing interests: The authors declare no conflict of Interest.
References
- 1.Bellos A, Mulholland K, O’Brien KL, Qazi SA, Gayer M, Checchi F. The burden of acute respiratory infections in crisis-affected populations: a systematic review. Confl Health. 2010;4:3. doi: 10.1186/1752-1505-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO. The global burden of disease: 2004 update [Internet] WHO; [cited 2016 May 11]. Available from: http://www.who.int/healthinfo/global_burden_disease/2004_report_update/en/ [Google Scholar]
- 3.Williams BG, Gouws E, Boschi-Pinto C, Bryce J, Dye C. Estimates of world-wide distribution of child deaths from acute respiratory infections. Lancet Infect Dis. 2002 Jan;2(1):25–32. doi: 10.1016/S1473-3099(01)00170-0. [DOI] [PubMed] [Google Scholar]
- 4.Nichols WG, Campbell AJP, Boeckh M. Respiratory Viruses Other than Influenza Virus: Impact and Therapeutic Advances. Clin Microbiol Rev. 2008 Apr 1;21(2):274–90. doi: 10.1128/CMR.00045-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mahony JB. Detection of Respiratory Viruses by Molecular Methods. Clin Microbiol Rev. 2008 Oct 1;21(4):716–47. doi: 10.1128/CMR.00037-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goldmann DA. Epidemiology and prevention of pediatric viral respiratory infections in health-care institutions. Emerg Infect Dis. 2001;7(2):249–53. doi: 10.3201/eid0702.010220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berry M, Gamieldien J, Fielding BC. Identification of New Respiratory Viruses in the New Millennium. Viruses. 2015 Mar 6;7(3):996–1019. doi: 10.3390/v7030996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nair H, Brooks WA, Katz M, Roca A, Berkley JA, Madhi SA, et al. Global burden of respiratory infections due to seasonal influenza in young children: a systematic review and meta-analysis. Lancet Lond Engl. 2011 Dec 3;378(9807):1917–30. doi: 10.1016/S0140-6736(11)61051-9. [DOI] [PubMed] [Google Scholar]
- 9.Nair H, Nokes DJ, Gessner BD, Dherani M, Madhi SA, Singleton RJ, et al. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. The Lancet. 2010 May 7;375(9725):1545–55. doi: 10.1016/S0140-6736(10)60206-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fendrick AM, Monto AS, Nightengale B, Sarnes M. The economic burden of non-influenza-related viral respiratory tract infection in the United States. Arch Intern Med. 2003 Feb 24;163(4):487–94. doi: 10.1001/archinte.163.4.487. [DOI] [PubMed] [Google Scholar]
- 11.Bawage SS, Tiwari PM, Pillai S, Dennis V, Singh SR. Recent Advances in Diagnosis, Prevention, and Treatment of Human Respiratory Syncytial Virus. Adv Virol [Internet] 2013. [cited 2016 May 11] Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3872095/ [DOI] [PMC free article] [PubMed]
- 12.Hayden F. Developing New Antiviral Agents for Influenza Treatment: What Does the Future Hold? Clin Infect Dis. 2009 Jan 1;48(Supplement 1):S3–13. doi: 10.1086/591851. [DOI] [PubMed] [Google Scholar]
- 13.Hayden FG. Advances in antivirals for non-influenza respiratory virus infections. Influenza Other Respir Viruses. 2013 Nov 1;7:36–43. doi: 10.1111/irv.12173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gill JM, Fleischut P, Haas S, Pellini B, Crawford A, Nash DB. Use of antibiotics for adult upper respiratory infections in outpatient settings: A national ambulatory network study. Fam Med. 2006 May;38(5):349–54. [PubMed] [Google Scholar]
- 15.Gonzales R, Malone DC, Maselli JH, Sande MA. Excessive Antibiotic Use for Acute Respiratory Infections in the United States. Clin Infect Dis. 2001 Sep 15;33(6):757–62. doi: 10.1086/322627. [DOI] [PubMed] [Google Scholar]
- 16.Pavia AT. Viral Infections of the Lower Respiratory Tract: Old Viruses, New Viruses, and the Role of Diagnosis. Clin Infect Dis. 2011 May 1;52(suppl 4):S284–9. doi: 10.1093/cid/cir043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li H, McCormac MA, Estes RW, Sefers SE, Dare RK, Chappell JD, et al. Simultaneous detection and high-throughput identification of a panel of RNA viruses causing respiratory tract infections. J Clin Microbiol. 2007 Jul;45(7):2105–9. doi: 10.1128/JCM.00210-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Finianos M, Issa R, Curran MD, Afif C, Rajab M, Irani J, et al. Etiology, seasonality and clinical characterization of viral respiratory infections among hospitalized children in Beirut, Lebanon. J Med Virol. 2016 Apr 1; doi: 10.1002/jmv.24544. n/a-n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wertheim HFL, Nadjm B, Thomas S, Agustiningsih A, Malik S, Diep NNT, et al. Viral and atypical bacterial aetiologies of infection in hospitalised patients admitted with clinical suspicion of influenza in Thailand, Vietnam and Indonesia. Influenza Other Respir Viruses. 2015 May 16; doi: 10.1111/irv.12326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Regamey N, Kaiser L, Roiha HL, Deffernez C, Kuehni CE, Latzin P, et al. Viral Aetiology of Acute Respiratory Infections With Cough in Infancy: A Community-Based Birth Cohort Study. Pediatr Infect Dis J [Internet] 2008 Jan; doi: 10.1097/INF.0b013e31815922c8. [cited 2015 Jul 20];PAP. Available from: http://content.wkhealth.com/linkback/openurl?sid=WKPTLP:landingpage&an=00006454-900000000-99976. [DOI] [PubMed] [Google Scholar]
- 21.Ali SA, Williams JV, Chen Q, Faori S, Shehabi A, Jundi EA, et al. Human metapneumovirus in hospitalized children in Amman, Jordan. J Med Virol. 2010 May 1;82(6):1012–6. doi: 10.1002/jmv.21768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shafik CF, Mohareb EW, Yassin AS, Amin MA, El Kholy A, El-Karaksy H, et al. Viral etiologies of lower respiratory tract infections among Egyptian children under five years of age. BMC Infect Dis. 2012;12:350. doi: 10.1186/1471-2334-12-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Al-Thani A, Azzam SB, Al-Sheik Abboubaker HM, Abdel-Hadi FG, Elsheikh M, Janahi IA. The role of human metapneumovirus in pediatric respiratory tract infection in Qatar. Future Virol. 2010 May;5(3):355–60. doi: 10.2217/fvl.10.13. [DOI] [Google Scholar]
- 24.Garcia J, Espejo V, Nelson M, Sovero M, Villaran MV, Gomez J, et al. Human rhinoviruses and enteroviruses in influenza-like illness in Latin America. Virol J. 2013;10:305. doi: 10.1186/1743-422X-10-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laguna-Torres VA, Sánchez-Largaespada JF, Lorenzana I, Forshey B, Aguilar P, Jimenez M, et al. Influenza and other respiratory viruses in three Central American countries. Influenza Other Respir Viruses. 2011 Mar 1;5(2):123–34. doi: 10.1111/j.1750-2659.2010.00182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Diaz J, Morales-Romero J, Pérez-Gil G, Bedolla-Barajas M, Delgado-Figueroa N, García-Román R, et al. Viral coinfection in acute respiratory infection in Mexican children treated by the emergency service: A cross-sectional study. Ital J Pediatr. 2015 Apr 18;41(1):33. doi: 10.1186/s13052-015-0133-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tregoning JS, Schwarze J. Respiratory Viral Infections in Infants: Causes, Clinical Symptoms, Virology, and Immunology. Clin Microbiol Rev. 2010 Jan 1;23(1):74–98. doi: 10.1128/CMR.00032-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Esper FP, Spahlinger T, Zhou L. RATE AND INFLUENCE OF RESPIRATORY VIRUS CO-INFECTION ON PANDEMIC (H1N1) INFLUENZA DISEASE. J Infect. 2011 Oct;63(4):260–6. doi: 10.1016/j.jinf.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Semple MG, Cowell A, Dove W, Greensill J, McNamara PS, Halfhide C, et al. Dual Infection of Infants by Human Metapneumovirus and Human Respiratory Syncytial Virus Is Strongly Associated with Severe Bronchiolitis. J Infect Dis. 2005 Feb 1;191(3):382–6. doi: 10.1086/426457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aberle JH, Aberle SW, Pracher E, Hutter H-P, Kundi M, Popow-Kraupp T. Single versus dual respiratory virus infections in hospitalized infants: impact on clinical course of disease and interferon-gamma response. Pediatr Infect Dis J. 2005 Jul;24(7):605–10. doi: 10.1097/01.inf.0000168741.59747.2d. [DOI] [PubMed] [Google Scholar]
- 31.Wilkesmann A, Schildgen O, Eis-Hübinger AM, Geikowski T, Glatzel T, Lentze MJ, et al. Human metapneumovirus infections cause similar symptoms and clinical severity as respiratory syncytial virus infections. Eur J Pediatr. 2006 Jul;165(7):467–75. doi: 10.1007/s00431-006-0105-4. [DOI] [PubMed] [Google Scholar]
- 32.Bezerra PGM, Britto MCA, Correia JB, Duarte M, do CMB, Fonceca AM, Rose K, et al. Viral and atypical bacterial detection in acute respiratory infection in children under five years. PloS One. 2011;6(4):e18928. doi: 10.1371/journal.pone.0018928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shinjoh M, Omoe K, Saito N, Matsuo N, Nerome K. In vitro growth profiles of respiratory syncytial virus in the presence of influenza virus. Acta Virol. 2000 Apr;44(2):91–7. [PubMed] [Google Scholar]