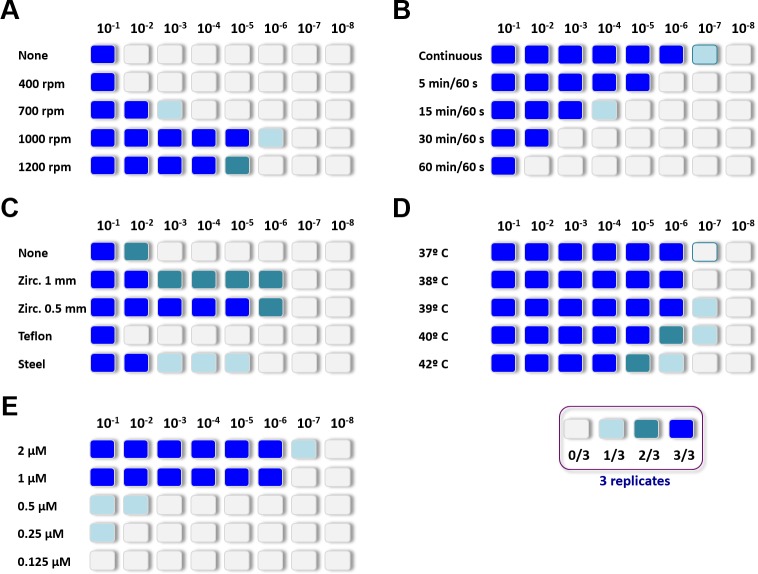
Fig 3. Optimization of PMSA parameters to increase propagation efficiency.
Variations of several operational parameters for PMSA were tested using serial dilutions of the L-seeded-PMSA (from 10−1 to 10−8) in a substrate composed of bank vole 109I rec-PrP complemented with dextran sulfate. For each parameter variation triplicates of the dilutions were included and the maximum dilution that showed protease-resistant rec-PrP was monitored by PK digestion and Western blot after one 24 h round of PMSA. Dilutions with detectable protease-resistant rec-PrP in each triplicate are represented in different shades of blue. Studied parameters include: (A) Shaking speed, ranging from no shaking (none) to 1,200 rpm; optimal propagation was achieved at 1,000 rpm; (B) Shaking/incubation intervals, from continuous shaking to cycles of 60 s shaking and 60 min of incubation, the optimal result was with continuous shaking; (C) Types of beads, several beads made of different materials or sizes were tested, some previously shown to improve propagation in PMCA [84, 85]. Similar amounts of each bead type were added to the dilutions: zirconium silicate 1.0 mm and 0.5 mm, polytetrafluoroethylene (PTFE) 1.8 mm and steel 1.0 mm. Dilutions without beads were also included. Propagation in the presence of zirconium silicate beads was significantly better, beads of PTFE or steel being similar to the absence of beads. Although 0.5 mm zirconium beads were more consistent than the 1.0 mm ones both reached 10−6 maximum dilutions and thus, 1.00 mm ones were chosen due to ease of handling; (D) Temperature variations in the range tested from 37 to 42°C, did not affect the propagation efficiency of seed greatly. However, propagation from the lowest dilutions was achieved at 39 and 40°C and so 39°C was chosen as optimum temperature setting; and (E) PrP concentration was evaluated in substrates varying from 0.125 μM to 2 μM. This is critical to obtain optimal amounts of material for structural studies with in vitro recombinant prion propagation methods. A balance is required between a high enough concentration to induce good propagation without wasting recombinant PrP and minimizing the amount of non-misfolded PrP remaining at the end of the reaction which will need to be eliminated prior to the structural studies. Below 1 μM PrP concentration was insufficient to efficiently propagate and the substrate with the highest protein concentration was best, so 2 μM was chosen as preferred concentration.