Fig 5. Propagation efficiency in PMSA analyzed by sedimentation and protease digestion.
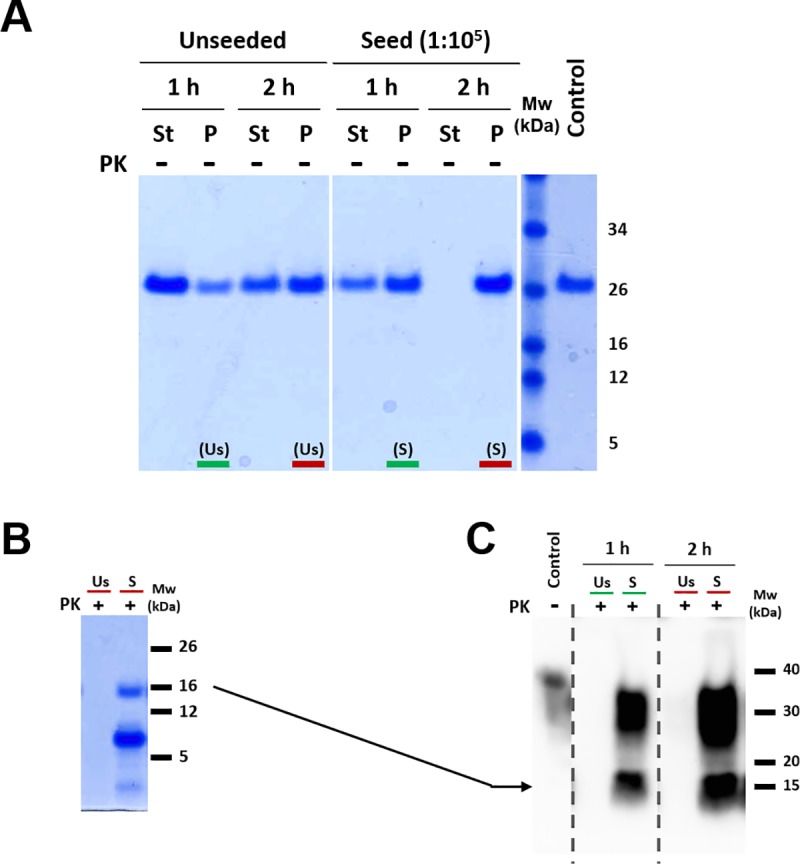
A) Total protein staining of the undigested pellet (P) vs. supernatant (St) fractions to monitor the amounts of aggregated and soluble rec-PrP in the course of PMSA. Fractions corresponding to 1 and 2 h from a 24 h-round of unseeded and seeded (1:100,000 dilution) PMSA reactions were collected at the indicated time points, in which all the PrP from the supernatant of the seeded sample was converted to insoluble material. Samples were centrifuged at 19,000 g for 15 min and supernatant and pellet (resuspended in a volume equal to that of the collected fraction) subjected to electrophoresis and stained for total protein with BlueSafe. The amount of aggregated PrP (pellet) and soluble PrP (supernatant) were compared in order to calculate conversion efficiency without protease digestion due to the possibility of protease-sensitive prions being an important part of the PMSA product. The unseeded reaction indicated the maximum amount of amorphous aggregates that can be formed after 2 h of the process was approximately 50% of the total PrP, which was subtracted from the aggregated fraction of the seeded reaction to estimate bona fide misfolding percentage. A highly diluted seed (10−5) was used to minimize its effect on driving the misfolding process towards the bona fide prion formation pathway at the expense of the formation of amorphous aggregates. Therefore, it is likely that bona fide prion propagation efficiency is higher than the 50% estimated based on the amount of amorphous aggregates formed in an unseeded reaction. Mw: molecular weight marker. Control: total PrP on the substrate at time 0, before PMSA. B) Total protein staining of protease digested PMSA products at 2 h. To differentiate samples containing only amorphous aggregates (non-infectious completely protease-sensitive) from samples containing bona fide recombinant prions (partially protease-resistant), samples form unseeded (Us) and seeded (S) reactions were collected after 2 h of PMSA and digested with PK (25 μg/ml) for 1h at 42°C. As expected, the unseeded reaction contained only PK-sensitive aggregates, while the seeded one showed the characteristic PK-resistant core that remains after amino-terminal digestion, indicating that at least part of the misfolded material formed in the seeded reaction were PK-resistant bona fide prions. Mw: molecular weight marker. C) Potential infectivity of unseeded and seeded samples by brain-PMCA. In order to confirm that the unseeded sample did not contain PK-sensitive bona fide prions able to propagate their conformation in brain-derived PrP whereas the seeded sample was, the two samples at 2 h were used as seeds to inoculate TgVole (1x) brain homogenate at 1:10 dilution and submitted to brain-PMCA. The PMCA products were PK-digested and visualized by Western blotting, which showed the presence of PK-resistant PrP for both seeded samples while there was no detectable propagation for the unseeded ones after 1 and 2 h PMSA. The high dilution of the original L-seeded-PMSA inoculum (10−5) protects against the possibility of being the original inoculum and not the propagated material, the result of brain PrPC misfolding, since the propagation capacity of L-seeded-PMSA in brain-PMCA did not reach 10−6 dilution. mAb: D18 (1:5,000). Mw: molecular weight marker. Control: undigested Tgvole (1x) PrP.
