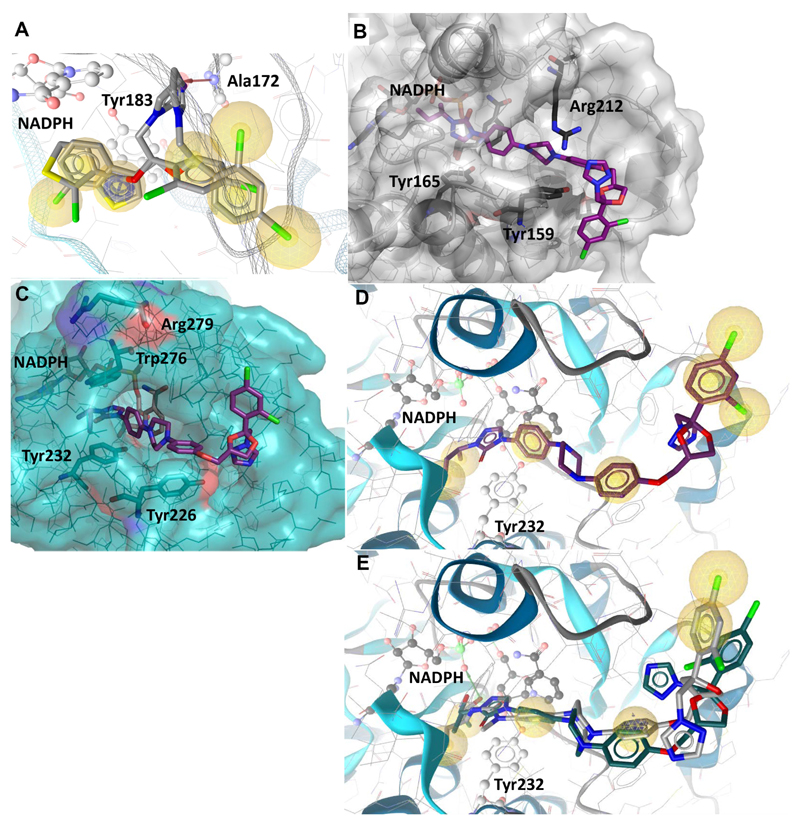
Fig. 5. Predicted binding of selected fungicides to 11β-HSD1 and 11β-HSD2.
(A) Representative binding poses of inhibitors in human 11β-HSD1 exemplified by tioconazole and sertaconazole. Important interactions for protein-ligand binding and the cofactor are shown in ball-and-stick style. Hydrophobic contacts between the ligand and the binding site are represented as yellow spheres, the HBA to Ala172 as red arrow and the aromatic stacking with Tyr183 as blue circle. (B) Binding mode of itraconazole docked into the homology model of human 11β-HSD2. (C) Itraconazole docked into the homology model of murine 11β-HSD2. Arg279 (equivalent to Arg212 in human 11β-HSD2) is pointing away from the binding pocket, while Trp276 blocks the binding pocket next to the cofactor NADPH. (D) Itraconazole docked into the homology model of murine 11β-HSD2. The ligand is not anchored via hydrogen bonds or charged interactions, only hydrophobic contacts (yellow spheres) are formed. (E) Posaconazole (grey) and OHI (green) docked into murine 11β-HSD2. Their hydroxyl groups form hydrogen bonds (green arrow) with the cofactor, which provides an explanation for their higher activity compared to itraconazole.