Abstract
Background
An increase in size of the aperture of the pelvis that must be spanned by pelvic floor support structures translates to an increase in the force on these structures. Prior studies have measured the bony dimensions of the pelvis, but the effect of changes in muscle bulk that may affect the size of this area are unknown.
Objectives
To develop a technique to evaluate the aperture size in the anterior pelvis at the level of the levator ani muscle attachments, and identify age-related changes in women with and without prolapse.
Study Design
This was a technique development and pilot case-control study evaluating pelvic magnetic resonance imaging (MRI) from 30 primiparous women from the Michigan Pelvic Floor Research Group MRI Data Base: 10 younger women with normal support, 10 older women with, and 10 older menopausal women without prolapse. Anterior pelvic area measurements were made in a plane that included the bilateral ischial spines and the inferior pubic point, approximating the level of the arcus tendineus fascia pelvis. Measurements of the anterior pelvic area, obturator internus muscles, and interspinous diameter were made by five independent raters from the Society of Gynecologic Surgeons Pelvic Anatomy Group that focused on developing pelvic imaging techniques, and evaluating inter-rater reliability. Demographic characteristics were compared across groups of interest using Wilcoxon rank sum test, Chi-square, or Fisher’s exact test where appropriate. Multiple linear regression models were created to identify independent predictors of anterior pelvic area.
Results
Per the study design, groups differed in age and prolapse stage. There were no differences in race, height, body mass index, gravidity or parity. Patients with prolapse had a significantly longer interspinous diameter, and more major (>50% of the muscle) levator ani defects when compared to both older and younger women without prolapse. Inter-rater reliability was high for all measurements (ICC=.96). The anterior pelvic area (cm2) was significantly larger in older women with prolapse compared to older (60±5.1 vs. 53±4.9, p=.004) and younger (60±5.1 vs. 52±4.6, p=.001) women with normal support. The young and older normal support women did not differ in anterior pelvic area (52±4.6 vs 53±4.9, p= 0.99). After adjusting for race and BMI, increased anterior pelvic area was significantly associated with the following: 1) being an older woman with prolapse (β = 6.61cm2, p=.004), and 2) IS distance (β = 4.52cm2, p=.004).
Conclusions
Older women with prolapse had the largest anterior area, suggesting that the anterior pelvic area is a novel measure to consider when evaluating women with prolapse. Interspinous diameter, and being an older woman with prolapse, was associated with a larger anterior pelvic area. This suggests that reduced obturator internus muscle size with age may not be the primary factor in determining anterior pelvic area, but pelvic dimensions such as interspinous diameter could play a role. The measurements were highly repeatable. The high ICC indicates that all raters were able to successfully learn the imaging software and perform measurements with high reproducibility.
Keywords: Pelvic organ prolapse, Magnetic resonance imaging, pelvic floor, aging
Condensation
The novel measurement of anterior pelvic area takes into consideration muscular and bony aspects of the pelvic floor and is larger in older women with prolapse.
Introduction
Pelvic organ prolapse (POP) is a common condition and carries up to a 20% lifetime risk of surgery.1–3 Currently, 200,000 procedures are performed annually, yet recurrence of prolapse is common,4 which increases the cost and morbidity of treatment.5, 6 Due to the increasing age of the general population, the number of operations for prolapse is anticipated to increase in the coming decades.7 Minimizing failures and complications will require understanding the pathophysiology of POP to develop interventions that may prevent its development and recurrence after treatment.
The cause of pelvic floor disorders has been shown to be multifactorial, with age, parity, mode of delivery and injury to the levator ani muscles all contributing to their development. 8–10 The size of the aperture in the pelvis that must be spanned by the pelvic floor has mechanistic importance. The larger the size of the aperture, the greater the force developed by the pressure differential between abdominal and atmospheric pressure (pressure × area = force, Figure 1a). Simply, the larger the aperture, the greater the force on the structures spanning it. Women with larger pelvic floor areas will have more force transferred to their pelvic floor, which could play a role in the development of prolapse. Existing studies 11–13 only look at the bones that make up this area, but the internal obturator muscles actually form the lateral margins of the anterior pelvic canal through which prolapse occurs. Whether or not age-related atrophy known to occur in these muscles14 increases the size of the aperture is not yet known.
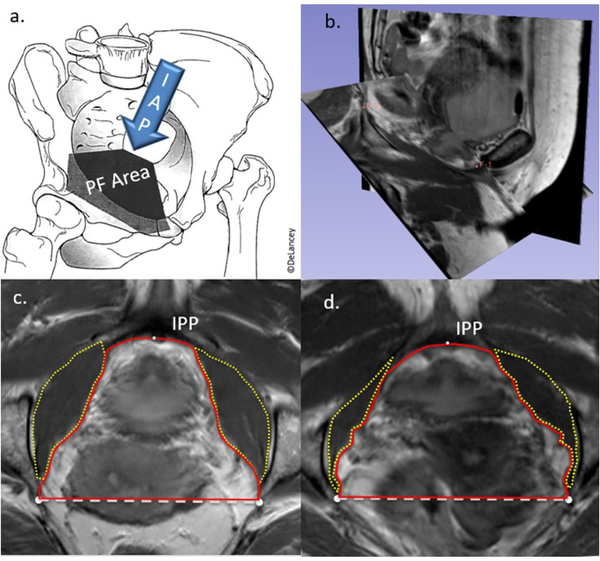
Figure 1:
a. Schematic of intraabdominal pressure (IAP) on the pelvic floor (PF) at the level of the attachment of the levator ani muscles. b. example of plane oblique to include the inferior pubic point (F-1) and the ischial spines (left noted with F-2). c. pelvic floor measurements made in the oblique plane in a woman with larger obturator internus muscles. IPP- inferior pubic point, yellow dotted lines outline the obturator internus muscles, red solid line represents the anterior pelvic area, white dashed line represents the interspinous diameter. d. Measurements in a patient with a larger anterior pelvic area and smaller obturator internus muscles
With current technology and software, traditionally acquired magnetic resonance imaging (MRI) can be reformatted into any oblique plane without loss of fidelity, to measure anatomy in customizable, clinically relevant planes, such as that which includes the attachment of the levator ani muscles to the arcus tendineus fascia pelvis. In this plane, the cross-sectional area of the anterior pelvic floor, including how it relates to its bony and muscular borders, can be measured (Figure 1b–d). The posterior pelvic area in this plane is formed by the sacrospinous ligament and sacrum, which would not be expected to have age-related changes in their positioning, and therefore was not included in this study.
The aims of this study were to evaluate a novel technique to measure the anterior pelvic area considering both bony and muscular pelvic borders, assess its inter-rater reliability, and evaluate the anterior pelvic area in younger and older primiparous women, with and without prolapse. The use of a customized, reformatted plane at the level of the levator ani muscle attachments in this study allows for evaluation of a novel and clinically relevant plane.
Materials and Methods
This was a technique development and pilot case-control study conducted utilizing images in the Michigan Pelvic Floor Research Group MRI Library. This library contains scans of approximately 1,400 women acquired during National Institute of Health funded research and includes roughly equal numbers of women with pelvic floor disorders and demographically similar asymptomatic volunteers. MRIs from 30 vaginally primiparous women were analyzed. Three groups were compared: 1) ten younger women (aged 18–39 years) who were recruited nine months after their first vaginal birth, 2) ten older (age≥47 years) women who did not have pelvic organ prolapse recruited as controls for mechanistic studies and, 3) ten older (age≥47 years) women who were recruited as prolapse cases for mechanistic studies (defined as the leading edge of the vagina at least one cm beyond the hymen on POP-Q exam). All patients had a uterus in situ and had not had prior pelvic floor surgery. The current study (HUM00144643) and parent studies (HUM00043445 and HUM00031520) were approved by University of Michigan Institutional Review Board. The calculation of sample size was performed estimating a 28% difference in anterior pelvic area based on prior work by Morris et al comparing obturator internus muscle volume between younger and older women. 14 Based on this calculation, eight subjects per group were needed to detect a 28% difference with 80% power using a two-sided chi-square test of independence and alpha of 0.05.
The imaging protocol has been described in prior publications.15–17 In brief, among older women tri-planar MRI images were obtained in the supine position during rest using a 3-T Philips Achieva scanner with a six-channel, phased-array coil. Resting images were used in this study. The imaging protocol for the younger women has also been described previously.16 For these women, multiplanar proton density 2D fast spin images of the pelvic organs were obtained with an echo time of 15 msec and a repetition time of 4 sec using a 1.5 Tesla superconducting magnet (Signa; General Electric Medical Systems, Milwaukee, WI) with version 5.4 software.
Using 3D imaging software, 3DSlicer (v 4.5.0, www.slicer.org, Brigham and Women’s Hospital, Boston, MA), the measurement plane was reformatted and oblique to approximate the attachments of the levator ani to the arcus tendinous fascia pelvis. The plane was defined using 3 points: the bilateral ischial spines, and the inferior pubic point (Figure 1b). In this plane, the anterior pelvic area (“anterior area”) was defined as the area inside the line between the ischial spines posteriorly, the medial edges of the obturator internus muscles bilaterally, and the inferior pubic point on the lower edge of the pubic symphysis anteriorly (Figure 1c). Interspinous diameter was defined as the length of the line between the two ischial spines as visualized in this plane.
Measurements were made by five independent raters from the Society for Gynecologic Surgeons Pelvic Anatomy Group. This group is focused on learning pelvic floor imaging modalities. Each rater was trained to use 3D Slicer software and make measurements over a series of screen-sharing conference calls. Conference calls included training sessions with leader demonstrated measurements. Participants were assigned practice measurements that were then reviewed to ensure proper measurement technique. Each rater logged screen shots of their work, and measurements and data points were recorded from Slicer. These measurements were then compared. Outlying measurements were evaluated and addressed by examining screen shots. These were reviewed and discussed with the individual rater, and any appropriate changes were made. After a high inter-rater reliability was achieved on a number of practice scans, the raters were able to proceed with study measurements. The intra-class correlation coefficient (ICC) was calculated as a measure of inter-rater reliability.
Demographic characteristics were compared across groups of interest using Wilcoxon rank sum test, Chi-square, or Fisher’s exact test where appropriate. The normality of the anterior pelvic area measurements was confirmed by reviewing skew, kurtosis, QQ plot, histogram, and the Shapiro-Wilk test. Linear regression models were created to identify independent predictors of the anterior area. All analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, N.C.). Statistical significance was set at the α = 0.05 level.
Results
As intended in our study design, younger women without prolapse differed significantly in age compared to both older groups of women with and without prolapse (29.9 IQR (24.4,32.9) vs. 56.0 IQR (52.8,59.3) Older Prolapse and 29.9 IQR (24.4,32.9) vs. 55.9 IQR (54.2,58.1) Older No Prolapse, p<.0001 for both). Groups were similar with regard to race, height, BMI, and gravidity. Maximum prolapse was significantly larger in the Older Prolapse group, by study design. Genital hiatus, prevalence of major levator defect, and levator defect score were all greater in the Older Prolapse group (Table 1). The ICC among the five examiners was 0.96 for the anterior pelvic area.
Table 1:
Demographics and study characteristics
| Young women n = 10 | Older women without prolapse n = 10 | Older women with prolapse n = 10 | p Overall* | |
|---|---|---|---|---|
| Age, years (IQR) | 29.9 (24.4, 32.9) | 55.9 (54.2, 58.1) | 56.0 (52.8, 59.3) | <.0001 |
| White race, n (%) | 10 (100.0) | 8 (80.0) | 9 (90.0) | 0.76 |
| Height, inches ± SD | 66.0 ± 2.7 | 65.3 ± 1.7 | 65.2 ± 1.6 | 0.64 |
| BMI, kg/m2 (IQR) | 22.4 (19.2, 32.6) | 28.0 (22.9, 32.0) | 27.0 (22.3, 33.5) | 0.06 |
| Gravidity, number (IQR) | 1.0 (1.0, 2.0) | 1.0 (1.0, 2.0) | 2.0 (1.0, 3.0) | 0.13 |
| Parity, number (IQR) | 1.0 (1.0, 1.0) | 1.0 (1.0, 1.0) | 1.0 (1.0, 1.0) | >.99 |
| Maximum Prolapse, cm (IQR) † | −2.5 (−2.5, −2.0) | −1.0 (−2.0, −1.0) | 2.5 (1.0, 4.0) | <.0001 |
| Genital Hiatus, cm (IQR) | 3.0 (3.0, 4.0) | 2.8 (2.0, 3.0) | 4.0 (3.5, 5.0) | 0.03 |
| Major Levator Defect, n (%) | 0 (0.0)6 | 2 (20.0) | 7 (70.0) | 0.003 |
| Levator defect score, number (IQR) | 0.0 (0.0, 0.0) | 1.5 (0.0, 2.0) | 4.0 (3.0, 4.0) | 0.0007 |
| Interspinous diameter, cm± SD | 10.4 ± 0.5 | 10.4 ± 0.4 | 11.1 ± 0.5 | 0.004 |
| OIM left, cm2± SD | 9.2 ± 1.3 | 8.4 ± 1.3 | 7.8 ± 2.1 | 0.19 |
| OIM right, cm2± SD | 8.5 ± 1.4 | 8.2 ± 1.5 | 7.9 ± 1.6 | 0.63 |
| OIM total, cm2± SD | 17.7 ± 2.6 | 16.6 ± 2.5 | 15.7 ± 3.7 | 0.33 |
Overall test for differences across study groups were performed using Fisher’s exact test for categorical variables, Kruskal Wallis test for non-normally distributed continuous variables, and ordinary least squares regression for normally distributed continuous
Maximum prolapse is the greatest value of either Ba or Bp on the POP-Q
IQR - interquartile range
SD - standard deviation
OIM - obturator internus muscle
The anterior pelvic floor area was significantly larger in the Older Prolapse group compared to the both groups without prolapse (Older Prolapse: 60.3 ± 5.1 cm2 vs. Younger: 51.5 ± 4.6 cm2, p=0.001, and Older Prolapse: 60.3 ± 5.1 cm2 vs. Older No Prolapse: 52.7 ± 4.9 cm2, p=0.004). There was no significant difference in the anterior area between younger women and older women without prolapse (p=0.86).
Interspinous diameter was 6% larger in the Older Prolapse group compared to both groups without prolapse (Table 1, Younger vs. Older Prolapse p=0.007, Older No Prolapse vs. Older Prolapse p=0.01). There were no differences in interspinous diameter between the Younger and Older No Prolapse group (p=0.96). Although the total cross-sectional area of the obturator internus muscles in this plane decreased with age, this was not statistically significant in this cohort of patients.
Bivariate linear regression showed that among the Older Prolapse group, maximum prolapse, area of the left obturator internus muscle, and interspinous diameter were each significantly associated with changes in anterior pelvic area (Table 2). The coefficient of determination (R2 value), which indicates the proportion of the variance in one value that is related to another, were highest for study group, maximum prolapse, and interspinous diameter, followed by left obturator internus muscle cross-sectional area (Table 2).
Table 2.
Bivariate associations of characteristics of women with anterior pelvic area
| Characteristic | Anterior Pelvic Area Estimate (95% CI) | R2 | p |
|---|---|---|---|
| Age, years | 0.15 (−0.02, 0.31) | 0.10 | 0.09 |
| White race | 1.74 (−6.03, 9.52) | 0.01 | 0.65 |
| Height, inches | 0.02 (−1.16, 1.21) | 0.01 | 0.97 |
| BMI, kg/m2 | 0.12 (−0.23, 0.47) | 0.02 | 0.48 |
| Gravidity, number | 0.96 (−1.44, 3.37) | 0.02 | 0.42 |
| Study group | 0.42 | ||
| Young women | REF | ||
| Older women without prolapse | 1.14 (−3.33, 5.60) | 0.61 | |
| Older women with prolapse | 8.80 (4.34, 13.26) | 0.0004 | |
| Maximum Prolapse, cm | 1.66 (0.95, 2.37) | 0.45 | <.0001 |
| Genital Hiatus, cm | 1.19 (−0.14, 2.52) | 0.11 | 0.08 |
| Major Levator Defect | 3.49 (−1.43, 8.42) | 0.07 | 0.16 |
| Levator defect score, number | 0.92 (−0.27, 2.11) | 0.08 | 0.12 |
| OIM left, cm2 | −1.46 (−2.79, −0.13) | 0.15 | 0.03 |
| OIM right, cm2 | −1.09 (−2.53, 0.44) | 0.07 | 0.16 |
| OIM total, cm2 | −0.72 (−1.47, 0.03) | 0.12 | 0.06 |
| Interspinous diameter, cm | 6.70 (3.57, 9.82) | 0.41 | 0.0001 |
Ordinary least squares regression
REF stands for reference group (younger women)
OIM total =the sum of both OIM cross sectional areas in the anterior pelvic area plane)
After controlling for race and BMI, multivariable linear regression revealed that Study Group, specifically being in the Older Prolapse group, was associated with an increase in anterior pelvic area, as was an increase in interspinous diameter (Table 3).
Table 3.
Multivariable linear regression models of anterior pelvic area
| Characteristic | β (cm2) | 95% CI | p |
|---|---|---|---|
| White race | 3.43 | (−1.60, 8.47) | 0.18 |
| BMI, kg/m2 | −0.12 | (−0.37, 0.14) | 0.37 |
| Study group | |||
| Young women | REF | REF | |
| Older women without prolapse | 2.31 | (−1.74, 6.36) | 0.26 |
| Older women with prolapse | 6.61 | (2.10, 11.13) | 0.004 |
| Interspinous diameter, cm | 4.52 | (1.43, 7.62) | 0.004 |
REF stands for reference group (younger women)
Comment
Principal findings
Abdominal pressure acts on the anterior pelvic floor, and an increase in the pelvic area leads to an increase in force that must be supported by pelvic floor and its component parts. The borders of the study plane include the tendinous arches of the levator ani and pelvic fascia; known to be involved in pelvic organ support.10 Because these critical structures lie on the inner surface of the internal obturator muscle, their course is influenced by its bulk that is known to decrease in size with age.14 In this primiparous population, the anterior pelvic area is 17% larger in women with prolapse than in similarly aged controls and younger women without prolapse. Both older and younger women without prolapse had similar anterior area measurements, indicating that this phenomenon is not related to age alone.
Results
The anterior pelvic area size depends on its bony and muscular borders. Prior studies have evaluated the size and shape of the pelvis as they related to pelvic floor disorders and found that specific muscular and bony features are associated with an increased risk of POP. Specifically, anterior-posterior pelvic dimensions have been associated with levator ani muscle injury11 and a wider transverse pelvic inlet has been associated with pelvic organ prolapse.12,13 With regard to the musculature of the pelvis, Morris et al. demonstrated that the volume of the obturator internus muscle decreases by approximately 28% due to age-related atrophy.14 What is unknown, however, is the degree to which this muscle atrophy affects the pelvic floor area.
In this study, the bony pelvis that forms the lateral borders of the anterior area played a significant role. The interspinous diameter was 6% longer in patients with prolapse compared to those with normal support, and was significantly associated with an increase in the anterior pelvic area on linear regression. Bivariate regression revealed that interspinous diameter accounts for 41% of the variation in the anterior area. This suggests that in this population, women with wider pelvises or longer interspinous diameter were more likely to be in the prolapse cohort. This finding is consistent with prior literature describing a larger interspinous diameter and transverse pelvic measures in patients with prolapse than in controls.12, 13, 18 However, not all literature is consistent with regard to this point. Stein et al. reported no difference in interspinous diameter, or any other bony dimensions, between women with and without prolapse.19 Racial differences in pelvic dimensions could also play a role, however some published data suggest that the interspinous diameter is 5% smaller in African American women20 while other studies show no difference in this measure amongst women of different races.21
The cross sectional area of the obturator internus muscles that border the anterior area in this plane was 11–15% smaller between the younger women and older women with prolapse, supporting prior findings of decreasing volume with age. However, at this small sample size it did not meet statistical significance in this study. In bivariate regression analysis, the size of the left obturator internus was significantly associated with a decrease in the anterior area, while the right was not, bringing the total obturator internus muscle area just above the level of statistical significance (p=0.06). This suggests that there could be a relationship that exists between obturator internus muscle size and anterior area, which may be detected with a larger sample size. While the variation in size of the obturator internus muscles with age explains about 12% the increase in anterior area size, interspinous diameter was the more important modifier of the anterior area, accounting for 41% of the variation.
Clinical Implications
In this study, we introduce a novel measurement of the pelvic floor, and it will take time and additional study to understand the clinical relevance of the findings. Prior studies have looked at the role of maternal birth canal capacity and the risk of levator injury,22 indicating that women with smaller capacity are more likely to sustain a birth injury. Our findings suggest that there may be a balance in forces between having a pelvic floor large enough to allow for injury free delivery while at the same time minimizing the force from intra-abdominal pressure over time. Future studies are needed to assess whether the increase in anterior pelvic area size is a function of muscle atrophy or injury, bone structure, or if it is an independent factor related to the development of prolapse.
Research Implications
Now that the technique has been developed and its repeatability established, mechanistic studies and investigations involving more diverse populations can be conducted to better understand how and why this larger area affects pelvic organ support. Although it is plausible, as we have suggested, that it relates to increased forces generated by abdominal pressure acting on this area, the mechanical changes of the attachment points for the levator ani muscles and pubocervical fascia may also play a role as well. Future studies will include a population more diverse in terms of race, parity, age, and presence of levator injury. Given that some bony dimensions in this plane were highly significant, we plan to also include anterior-posterior and posterior pelvic area dimensions going forward. Future studies will also look more closely at impact that the size of the obturator internus muscle has on the development of prolapse.
Strengths and Limitations
This evaluation of a new parameter that assesses the changes in the area spanned by the pelvic floor, accounting for the muscular structures of the pelvic sidewall, adds to our knowledge of anatomical alterations present in women with prolapse. It also adds information by creating a novel measurement of the pelvic floor that factors in both bony and muscular features of the pelvis. Prior studies that have measured the bony landmarks near the attachments of the levator muscles have not been able to capture the effect of the obturator internus muscle.13, 18–20 The advantage of evaluating the pelvic floor in this novel, oblique plane is that we are able to measure the cross-sectional area of the pelvic floor at the level at which levator injury and paravaginal separation occurs, while accounting for the role that both muscular and boney components play.
Strengths of this study include our ability to measure a new, mechanistically relevant phenomenon. The inter-rater reliability of the measurements in our study was high, indicating that all reviewers from various national and international institutions were able to learn the software and measurement techniques to achieve reproducible results.23 Despite the relatively small number of women in each cohort, we were able to identify statistically significant relationships in the anterior pelvic area and other dimensions of the pelvis.
Due to a small sample size however, there is the potential for type 2 error in identifying relationships between some of the other pelvic parameters, such as the obturator internus muscle area and the anterior area. Sample size also limited the number of variables that could be included in the final linear regression model. Additionally, our cohort was primarily white/Caucasian, and studies in more racially diverse populations are needed to establish the broader generalizability of our findings.
Conclusions
In conclusion, we describe a novel measurement of the pelvic floor that relates to the amount of force it must bear and factors in both muscular and bony differences between patients with and without prolapse over time. Women with prolapse have the larger anterior area, largely influenced by the interspinous diameter, leading to an increase in force on the pelvic floor. Understanding this force on the pelvic floor, and what affects it, is key to developing clinical interventions for prevention and treatment of pelvic organ prolapse. Future studies could help clarify these relationships in a larger and more diverse population.
AJOG at a Glance.
This study was conducted to develop and test a new technique for measuring the pelvic floor while measuring changes in the anterior pelvic area through which prolapse occurs with age among primiparous women with and without prolapse.
We found that the anterior pelvic area is larger in older women with prolapse than in younger or older women without prolapse. The largest influence on this area is interspinous diameter.
This adds a novel measurement in a mechanistically relevant plane which takes into consideration both bony and muscular landmarks in the pelvic floor.
Acknowledgments
Funding: Supported by National Institutes of Health (NIH) ORWH SCOR grant P50 HD044406, the Eunice Kennedy Shriver National Institute of Child Health and Human Development R01 HD038665 and National Institute of Diabetes, Digestive, and Kidney Diseases R01 DK51405. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of interest summary: The authors report no conflict of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Olsen AL, Smith VJ, Bergstrom JO, Colling JC, Clark AL. Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstetrics and gynecology 1997;89:501–6. [DOI] [PubMed] [Google Scholar]
- 2.Hendrix SL, Clark A, Nygaard I, Aragaki A, Barnabei V, McTiernan A. Pelvic organ prolapse in the Women’s Health Initiative: gravity and gravidity. American journal of obstetrics and gynecology 2002;186:1160–6. [DOI] [PubMed] [Google Scholar]
- 3.Wu JM, Matthews CA, Conover MM, Pate V, Jonsson Funk M. Lifetime risk of stress urinary incontinence or pelvic organ prolapse surgery. Obstetrics and gynecology 2014;123:1201–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Friedman T, Eslick GD, Dietz HP. Risk factors for prolapse recurrence: systematic review and meta-analysis. International urogynecology journal 2018;29:13–21. [DOI] [PubMed] [Google Scholar]
- 5.Jelovsek JE, Barber MD, Brubaker L, et al. Effect of Uterosacral Ligament Suspension vs Sacrospinous Ligament Fixation With or Without Perioperative Behavioral Therapy for Pelvic Organ Vaginal Prolapse on Surgical Outcomes and Prolapse Symptoms at 5 Years in the OPTIMAL Randomized Clinical Trial. Jama 2018;319:1554–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nygaard I, Brubaker L, Zyczynski HM, et al. Long-term outcomes following abdominal sacrocolpopexy for pelvic organ prolapse. Jama 2013;309:2016–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu JM, Kawasaki A, Hundley AF, Dieter AA, Myers ER, Sung VW. Predicting the number of women who will undergo incontinence and prolapse surgery, 2010 to 2050. American journal of obstetrics and gynecology 2011;205:230.e1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nygaard I, Barber MD, Burgio KL, et al. Prevalence of symptomatic pelvic floor disorders in US women. Jama 2008;300:1311–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sze EH, Sherard GB 3rd, Dolezal JM. Pregnancy, labor, delivery, and pelvic organ prolapse. Obstetrics and gynecology 2002;100:981–6. [DOI] [PubMed] [Google Scholar]
- 10.DeLancey JOL, Morgan DM, Fenner DE, et al. Comparison of Levator Ani Muscle Defects and Function in Women With and Without Pelvic Organ Prolapse. Obstetrics & Gynecology 2007;109:295–302. [DOI] [PubMed] [Google Scholar]
- 11.Berger MB, Doumouchtsis SK, Delancey JO. Are bony pelvis dimensions associated with levator ani defects? A case-control study. International urogynecology journal 2013;24:1377–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sze EH, Kohli N, Miklos JR, Roat T, Karram MM. Computed tomography comparison of bony pelvis dimensions between women with and without genital prolapse. Obstetrics and gynecology 1999;93:229–32. [DOI] [PubMed] [Google Scholar]
- 13.Handa VL, Pannu HK, Siddique S, Gutman R, VanRooyen J, Cundiff G. Architectural differences in the bony pelvis of women with and without pelvic floor disorders. Obstetrics and gynecology 2003;102:1283–90. [DOI] [PubMed] [Google Scholar]
- 14.Morris VC, Murray MP, Delancey JO, Ashton-Miller JA. A comparison of the effect of age on levator ani and obturator internus muscle cross-sectional areas and volumes in nulliparous women. Neurourology and urodynamics 2012;31:481–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen L, Lisse S, Larson K, Berger MB, Ashton-Miller JA, DeLancey JO. Structural Failure Sites in Anterior Vaginal Wall Prolapse: Identification of a Collinear Triad. Obstetrics and gynecology 2016;128:853–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DeLancey JO, Kearney R, Chou Q, Speights S, Binno S. The appearance of levator ani muscle abnormalities in magnetic resonance images after vaginal delivery. Obstetrics and gynecology 2003;101:46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DeLancey JO, Morgan DM, Fenner DE, et al. Comparison of levator ani muscle defects and function in women with and without pelvic organ prolapse. Obstetrics and gynecology 2007;109:295–302. [DOI] [PubMed] [Google Scholar]
- 18.Brown KM, Handa VL, Macura KJ, DeLeon VB. Three-dimensional shape differences in the bony pelvis of women with pelvic floor disorders. International urogynecology journal 2013;24:431–9. [DOI] [PubMed] [Google Scholar]
- 19.Stein TA, Kaur G, Summers A, Larson KA, DeLancey JO. Comparison of bony dimensions at the level of the pelvic floor in women with and without pelvic organ prolapse. American journal of obstetrics and gynecology 2009;200:241.e1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baragi RV, Delancey JO, Caspari R, Howard DH, Ashton-Miller JA. Differences in pelvic floor area between African American and European American women. American journal of obstetrics and gynecology 2002;187:111–5. [DOI] [PubMed] [Google Scholar]
- 21.Handa VL, Lockhart ME, Fielding JR, et al. Racial differences in pelvic anatomy by magnetic resonance imaging. Obstetrics and gynecology 2008;111:914–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tracy PV, DeLancey JO, Ashton-Miller JA. A Geometric Capacity-Demand Analysis of Maternal Levator Muscle Stretch Required for Vaginal Delivery. Journal of biomechanical engineering 2016;138:021001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977;33:159–74. [PubMed] [Google Scholar]