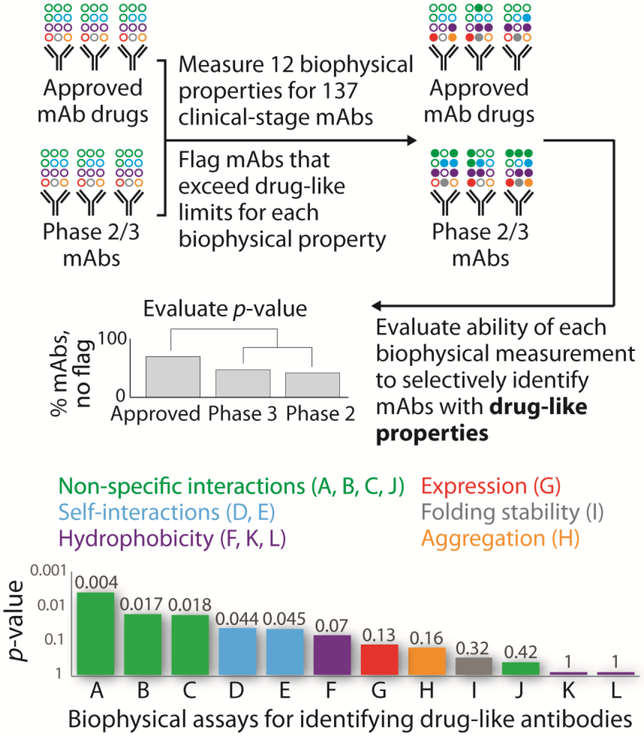
Figure 2. Approved mAb drugs are more specific on average than mAbs in clinical trials.
Jain and colleagues performed a broad study of the biophysical properties of clinical-stage mAbs using twelve assays to identify the most important biophysical determinants of drug-like antibodies [7••]. Notably, measurements of antibody specificity (non-specific binding and self-association) were the only ones to statistically differentiate between approved antibody drugs and antibodies in clinical trials. This finding suggests that approved antibody drugs are, on average, more specific than those in clinical trials and highlights the importance of using specificity assays to guide the selection of antibodies with drug-like properties. The reported assays are: (A) baculovirus particle (BVP), (B) ELISA (panel of six biomolecules), (C) polyspecificity reagent (PSR), (D) clone self-interaction by biolayer interferometry (CSI-BLI), (E) affinity-capture self-interaction nanoparticle spectroscopy (AC-SINS), (F) salt gradient affinity-capture self-interaction nanoparticle spectroscopy (SGAC-SINS), (G) expression titer in HEK cells, (H) aggregation (accelerated stability), (I) folding stability (melting temperature of Fab), (J) cross-interaction chromatography (CIC), (K) hydrophobic interaction chromatography (HIC), and (L) standup monolayer adsorption chromatography (SMAC). It is important to note that poor performance in a single biophysical assay does not preclude the successful development of a therapeutic antibody.