Introduction
Vascular endothelial growth factor (VEGF) is a vital factor needed for proper endothelial cell survival, and it is increasingly recognized as an important part of podocyte cellular physiology.1 The disruption of VEGF signaling has been known as a therapeutic target in processes that involve blood vessel proliferation (angiogenesis), and as such, systemically the drugs inhibiting VEGF function are used as anti-neoplastic anti-angiogenesis adjunctive treatments.2 The systemic effects of VEGF blockade were first realized with findings of severe hypertensive crisis (due to nitric oxide signaling depletion) and overt proteinuria. Findings of thrombotic microangiopathy (TMA), increased proteinuria, and multiple causes of nephrotic syndrome were published by many experts in the burgeoning field of onco-nephrology.2, 3 It has been notable that agents blocking mammalian target of rapamycin and the tyrosine kinase pathway (TKI) produce similar renal side effects as systemic VEGF blockade.4 Physiologically, preeclampsia demonstrated similar renal effects with soluble fms-like tyrosine kinase-1–induced depletion of VEGF.5
Intravitreal use of VEGF antagonists started off label with bevacizumab, and progressed with use of aflibercept (more potent), and ranibizumab (less potent) agents both given approval for age-related macular degeneration and diabetic retinopathy by the U.S. Food and Drug Administration.6
Intravitreal VEGF blocking agents have been shown to be absorbed,7 systemic effects, such as hypertension and renal disease, have been reported, including glomerular disease.6, 8, 9,S1–S3 We report a 96-year-old elderly woman who has been on ranibizumab, then bevacizumab, then aflibercept for progressive age-related macular degeneration since 2007. She at first had marginal amounts of proteinuria, between 2017 and 2018, the patient developed high-grade nephrotic-range proteinuria and a faster rate of progression of renal dysfunction. A renal biopsy disclosed an unexpected finding of collapsing focal and segmental sclerosis (FSGS), previously reported with systemic VEGF inhibitors and TKI.S4
Case Presentation
Our patient is a 96-year-old White woman with history of nephrolithiasis, colon cancer, and a wedge resection of the left middle lobe of kidney that had shown both no malignancy and no evidence of glomerular disease in 2001. She was started on anti-VEGF agents in 2007 when she was 84 years old. She also had a known history of hypertension and nephrolithiasis that required removal of the kidney stones via lithotripsy once.
She had a prior history of colon cancer, which was surgically resected in 2001, and a suspicious renal lesion was biopsied (a small lesion in the left middle lobe of kidney was resected) and found to have no malignancy. Importantly, the pathology reading from the kidney showed intact glomeruli without any evidence of systemic sclerosis or prominent hypertensive changes in her glomeruli. Renal function remained at baseline after the small resection: serum creatinine was between 1.0 and 1.1 between 2001 and 2007 without much fluctuation. After glaucoma and age-related macular degeneration were diagnosed in 2007, anti-VEGF therapy was started for severe age-related macular degeneration of her left eye. Initially, ranibizumab was started at 0.5 mg in the right eye (OD) given every 2 to 4 weeks depending on the needs with close monitoring. She underwent extensive numbers of injections in an effort to ameliorate the eyesight in her right eye and ultimately in both her right and left eyes (OS). She received 19 injections of ranibizumab (Lucentis) 0.5 mg, then had 54 injections of bevacizumab (Avastin) 1.25 mg, and finally had 75 injections of aflibercept (Eylea) 2 to 4 mg. No other nephrotoxic agents, including nonsteroidal anti-inflammatory agents or bisphosphonates,S5 were recorded or reported by the patients, and she did not develop diabetes mellitus type 2 during her treatment course. The patient’s medications include amlodipine, benazepril, brimonidine/timolol eye drops, cholecalciferol, furosemide, metoprolol, saliva substitute, simvastatin, spironolactone, and coumadin.
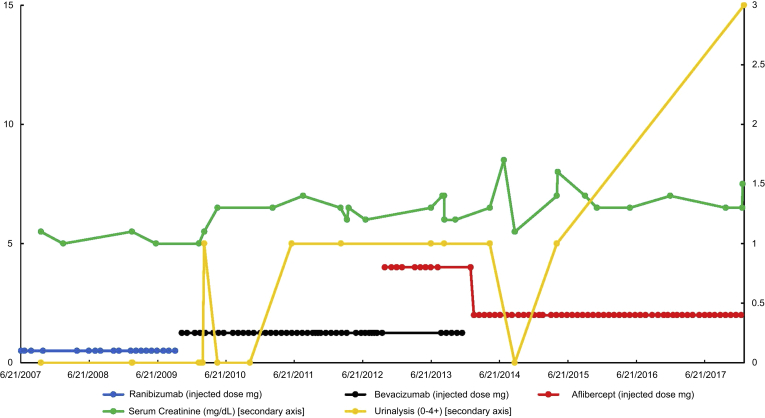
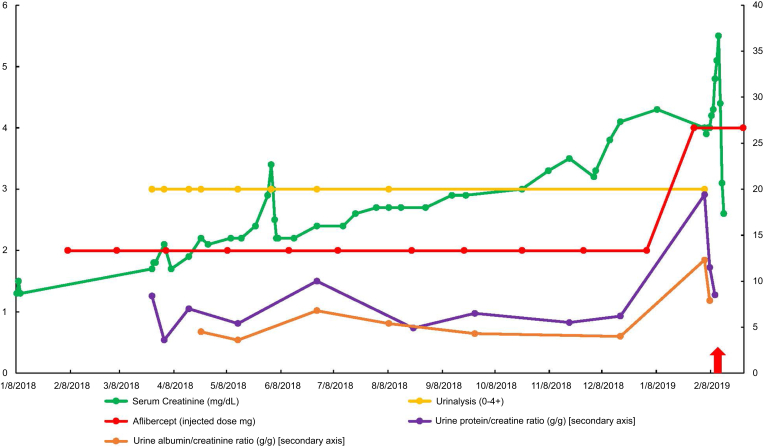
Initially, proteinuria started developing between 2010 and 2012 at 1+ on urinalysis, that was not present before. A rise in serum creatinine from 1.1–1.3 to 1.4–1.5 was noted with concomitant proteinuria. Blood pressure did not rise significantly in this case, but she continued to take her usual antihypertensive regimen. More pronounced proteinuria was noted in 2017 with a rise in urinalysis proteinuria to 3+. Between 2017 and 2018, urine protein-to-creatinine ratios were quantified at 3.6 to 8.4 g protein/g creatinine (urine albumin/creatinine ratios of 4–5 g albumin/g creatinine), which rose to a peak of 19.6 g protein/g creatinine. Her serum creatinine began to climb to 2 to 3 mg/dl between 2017 and 2018. Figures 1 and 2 demonstrate the progression of kidney function decline, proteinuria, and the type and doses of VEGF antagonists being given.
Figure 1.
The progression of serum creatinine, urinalysis proteinuria by time (including information of dose of ranibizumab, bevacizumab, and aflibercept injected between 2007–2017).
Figure 2.
The progression of serum creatinine, urinalysis proteinuria, urine protein/creatinine ratio, and urine albumin/creatinine ratio by time (including information of the dose of aflibercept injected between 2018–2019).
The patient underwent proteinuria workups that showed a slight elevation in kappa to lambda ratio of 2.8 to 3.3, but without findings of a monoclonal gammopathy. Her serological workup was unremarkable otherwise. She ultimately had a native kidney biopsy in December 2018, and the pathology results showed “collapsing pattern focal and segmental sclerosis, arterial nephrosclerosis was noted, and mild tubulointerstitial scarring.” The light microscopy contained 8 glomeruli, 3 of which were completely sclerotic. Of the remaining glomeruli, several have advanced lesions of segmental sclerosis and 1 or 2 have very prominent podocyte hypertrophy with numerous intracellular lipid vacuoles and protein droplets. This podocyte hypertrophy is characteristic of collapsing glomerulopathy, and no crescents were found. Congo red stain was done and was negative on light microscopy. Immunofluorescence was not positive for light or heavy chains of Igs. Electron microscopy was not able to be performed, as the biopsy showed only fragments of the corticomedullary junction. HIV was checked and found to be negative due to collapsing FSGS diagnosis. Figure 3 shows native kidney biopsy details from 2019.
Figure 3.
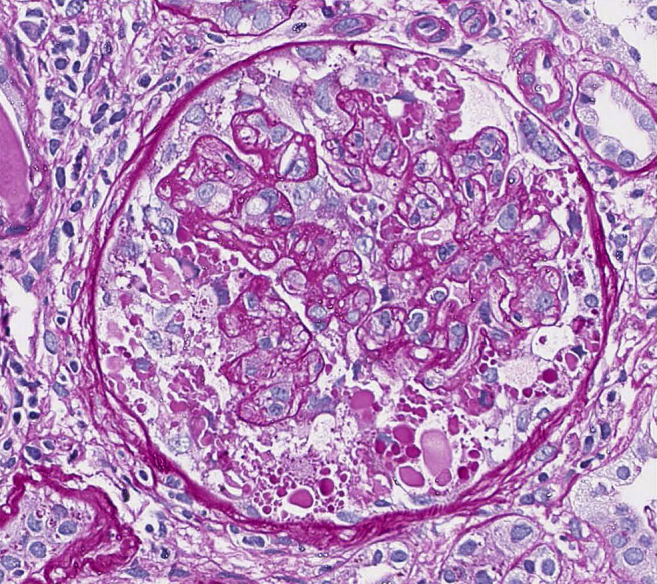
Native kidney biopsy findings in 2019 demonstrating collapsing focal and segmental sclerosis (hematoxylin and eosin, original magnification ×40 light microscopy).
The patient was White, not African American; this and her advanced age made genetic causes of FSGS much less likely. This is also in addition to renal tissue without scarring or FSGS findings in 2001. No viral prodrome or infection with cytomegalovirus, Epstein-Barr virus, or parvovirus was suspected on clinical grounds and no evidence was found of these infections serologically.
Serum VEGF-A level was obtained because the patient did not have many of the classic risk factors for FSGS, and the workup for the initially suspected amyloidosis was negative. Because a prior renal lobe resection pathology did not show these findings in 2001, the suspicion of intravitreal injections causing nephrotic-range proteinuria and FSGS was high. The VEGF-A level was obtained given suspicion of intravitreal anti-VEGF agent toxicity, and this level was found to be at <31 pg/ml (reference range 31–86 pg/ml), which was below the detection range. Of note, this blood test was obtained while the patient was still getting aflibercept injections.
At this point, the initial concern for intravitreal VEGF agent absorption and suppression of endogenous VEGF had a biochemical correlate.4, 6, 7 There was a full discussion with the patient and she declined to stop the intravitreal injections for fear of losing her eyesight. A trial of steroids and mycophenolate was initiated without positive effect on renal function or proteinuria, and she was transitioned to hemodialysis in February 2019.
Discussion
We discuss a case in which incidental biopsy data were obtained 6 years before the initiation of intravitreal VEGF blockade showing no hypertensive nephrosclerosis and no evidence of podocyte disruption or glomerulosclerosis. In the severe age-related macular degeneration treated in this patient, successively stronger agents were given at increasing doses to try preserving vision. Proteinuria developed at a low grade, and renal function declined slowly at first. Between 2017 and 2018 (10 years after initiating these injections), proteinuria became high grade, and renal function worsened more quickly. A renal biopsy ultimately showed glomerular disease, which was previously reported in TKI use and systemic VEGF blockade,S4 but had not been previously seen in patients with intravitreal VEGF blockade.6 This case illustrated a novel phenotype of glomerular disease and proteinuria ostensibly induced and/or worsened by intravitreal VEGF blockade. A prior normal native kidney biopsy in 2001 and findings of VEGF suppression in 2018 along with the worsening renal function, proteinuria, and native kidney biopsy-proven collapsing FSGS supported the role of intravitreal VEGF blockade in the development of this case of nephrotic syndrome.6, 7,S2
Despite multiple studies that only showed “no change in renal function and no proteinuria category (Kidney Disease Improving Global Outcomes A1–A3) with use of these agents,”S6,S7 there is evidence that some patients undergo drastic changes in renal function, hypertension control, and proteinuria, as reported in Bagheri et al.S8 It is becoming increasingly clear that like the phenotype seen in preeclampsia, VEGF depletion can be more disruptive to renal function for certain patients with environmental and genetic attributes making them more sensitive than others.5, 6 Given reports of systemic effects and increased mortality seen in population studies, a large-scale study accounting for proteinuria change as a continuous variable while monitoring drug and systemic VEGF levels is needed.6, 8, 9,S1,S2 Table 1 reviews other published cases of biopsy-confirmed glomerular disease associated with intravitreal anti-VEGF agent use.6,S2,S9–S13
Table 1.
Glomerular disease with intravitreal VEGF blockade
| Reference no. | n | Age(s) | Gender | Agent used | Finding |
|---|---|---|---|---|---|
| 6, S2 | 1 | 82 | F | Bev, Ran | De novo MCD |
| S9 | 2 | 52, 67 | 2 M | Bev | MGN, TMA |
| S10 | 1 | 74 | M | Bev | MGN |
| S11 | 1 | 77 | F | Ran | TMA |
| S12 | 1 | 54 | M | Bev | Relapsed MCD |
| S13 | 1 | 16 | F | Bev | Relapsed MCD |
| CC | 1 | 96 | F | Bev, Ran, Aflib | Collapsing FSGS |
#, number; Aflib, aflibercept; Bev, bevacizumab; CC, current case; F, female; FSGS, focal and segmental sclerosis; M, male; MCD, minimal change disease; MGN, membranous, glomerulonephritis, n, number of patients; Ran, ranibizumab; TMA, thrombotic microangiopathy; VEGF, vascular endothelial growth factor.
The type of lesion seen in this case report is unusual given the molecular biology of VEGF blockade and TKI blockade. These 2 agents should involve different arms of the C-Maf–inducing protein (C-MIP) and REL-A (v-rel avian reticuloendotheliosis viral oncogene homolog A) pathways. Systemic or intravitreal VEGF blockade should increase Rel-A and induce a proinflammatory signal through the nuclear factor kappa light chain enhancer of activated B cells, resulting in TMA. TKI agents act by blocking Rel-A and increase C-Maf–inducing protein (C-MIP), which alters the cell cytoskeleton and results in minimal change disease or FSGS (MCNS).S14 This was the observation made in the pioneering work of Izzedine et al. published in Kidney International.S15
The clinical reality involves overlap between TMA and other etiologies of nephrotic syndrome between VEGF blockers and TKI agents.2, 6,S2–S4,S16 Subsequent studies showed cases of minimal change disease or FSGS rather than TMA with VEGF blockade, and cases of TMA with TKI use.2, 6,S2–S4,S15–S16 Later studies compiled several cases of MCNS with intravitreal and systemic VEGF blockade.2, 6,S2,S4 So far, intravitreal VEGF blockade associated with nephrotic syndrome seems to have more reports of minimal change disease or FSGS than TMA. Although it is important to mention that some cases link ischemia from TMA with collapsing FSGS,S17 from the small number of reported cases, we feel it is premature to conjecture why TMA is not observed more often with intravitreal VEGF blockade. See Table 2 for a summary of teaching points of this report.2, 6,S2,S4
Table 2.
Teaching points
| 1. Systemic VEGF blockade is known to result in hypertension, proteinuria, and renal injury. |
| 2. Intravitreal anti-VEGF injections have been shown to be absorbed and inhibit systemic VEGF. |
| 3. Intravitreal anti-VEGF agents can cause TMA and nephrotic syndrome. |
| 4. Patients on intravitreal anti-VEGF therapy should be monitored for proteinuria, particularly if they have it at baseline. |
TMA, thrombotic microangiopathy; VEGF, vascular endothelial growth factor.
Disclosure
All the authors declared no competing interests.
Acknowledgments
This research work does not contain human subject research material, as it is an individual anonymized case report. International review board–permission was not applied for as it is not required for individual case reports in our institution (University of California Los Angeles, Los Angeles, CA). Consent was obtained from the patient, on condition that the no identifiable data be published, and patient consent was documented.
Footnotes
Supplementary References.
Supplementary Material
References
- 1.Henao D.E., Cadavid A.P., Saleem M.A. Exogenous vascular endothelial growth factor supplementation can restore the podocyte barrier-forming capacity disrupted by sera of preeclamptic women. J Obstet Gynaecol Res. 2013;39:46–52. doi: 10.1111/j.1447-0756.2012.01889.x. [DOI] [PubMed] [Google Scholar]
- 2.Hanna R.M., Lopez E., Wilson J. Minimal change disease onset observed after bevacizumab administration. Clin Kidney J. 2016;9:239–244. doi: 10.1093/ckj/sfv139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Izzedine H., Sene D., Hadoux J. Thrombotic microangiopathy related to anti-VEGF agents: intensive versus conservative treatment? Ann Oncol. 2011;22:487–490. doi: 10.1093/annonc/mdq743. [DOI] [PubMed] [Google Scholar]
- 4.Hanna R., Yanny B., Arman F. Everolimus worsening chronic proteinuria in patient with diabetic nephropathy post liver transplantation. Saudi J Kidney Dis Transpl. 2019;30:989–994. doi: 10.4103/1319-2442.265481. [DOI] [PubMed] [Google Scholar]
- 5.Thadhani R., Hagmann H., Schaarschmidt W. Removal of soluble Fms-like tyrosine kinase-1 by dextran sulfate apheresis in preeclampsia. J Am Soc Nephrol. 2016;27:903–913. doi: 10.1681/ASN.2015020157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hanna R.M., Barsoum M., Arman F. Nephrotoxicity induced by intravitreal vascular endothelial growth factor inhibitors: emerging evidence. Kidney Int. 2019;96:572–580. doi: 10.1016/j.kint.2019.02.042. [DOI] [PubMed] [Google Scholar]
- 7.Avery R.L., Castellarin A.A., Steinle N.C. Systemic pharmacokinetics and pharmacodynamics of intravitreal aflibercept, bevacizumab, and ranibizumab. Retina. 2017;37:1847–1858. doi: 10.1097/IAE.0000000000001493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hanhart J., Comaneshter D.S., Freier Dror Y. Mortality in patients treated with intravitreal bevacizumab for age-related macular degeneration. BMC Ophthalmol. 2017;17:189. doi: 10.1186/s12886-017-0586-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hanhart J., Comaneshter D.S., Freier-Dror Y. Mortality associated with bevacizumab intravitreal injections in age-related macular degeneration patients after acute myocardial infarct: a retrospective population-based survival analysis. Graefes Arch Clin Exp Ophthalmol. 2018;256:651–663. doi: 10.1007/s00417-018-3917-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.