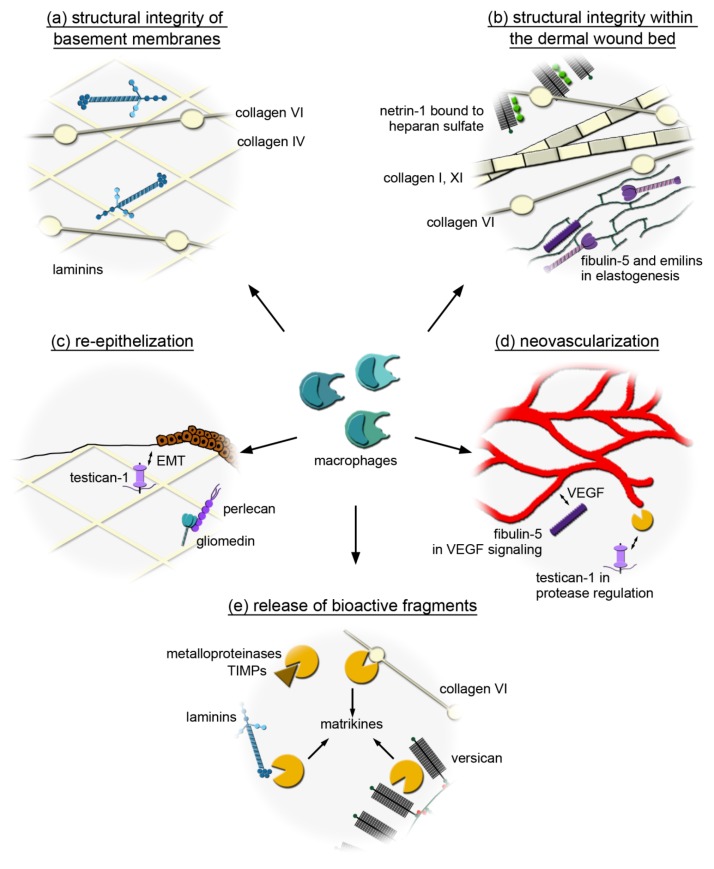
Figure 6.
Potential wound healing mechanisms involving the macrophage-derived extracellular matrix (ECM). (a) Macrophages could produce collagen IV, VI and laminins to provide their own substrate and/or bridging proteins for their adhesion to basement memranes. (b) Macrophages may promote the formation of a microfibrillar network within the wound by producing the beaded filament forming collagen VI. Collagen XI may be utilized by macrophages to interact with fibrillar collagens and support their organization while macrophage-derived netrin-1 may provide guidance signals to organize the wound. Macrophages may secrete fibulin-5 and EMILINs to act as a scaffold protein that organize and link elastic fibres. (c) Macrophage-derived gliomedin that binds to perlecan might participate in basement membrane formation/stabilization and influence keratinocytes to regulate epithelial wound closure. By producing testican-1, which is a target of TGF-β and induces epithelial-to-mesenchymal transition (EMT), macrophages may support epithelial wound closure. (d) Macrophage-derived testican-1 may also regulate metalloproteinase activity to promote the degradation of provisional wound matrix supporting vascularization, while fibulin-5 synthetized by macrophages may regulate angiogenesis by modulating VEGF signaling. (e) Macrophages may express proteases (A disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS2), MMP9, MMP1) and their inhibitors (TIMP1–3) to generate bioactive ECM fragments (matrikines) and may also be a source of ECM molecules that harbor these bioactive fragments (collagen VI, laminins, versican).