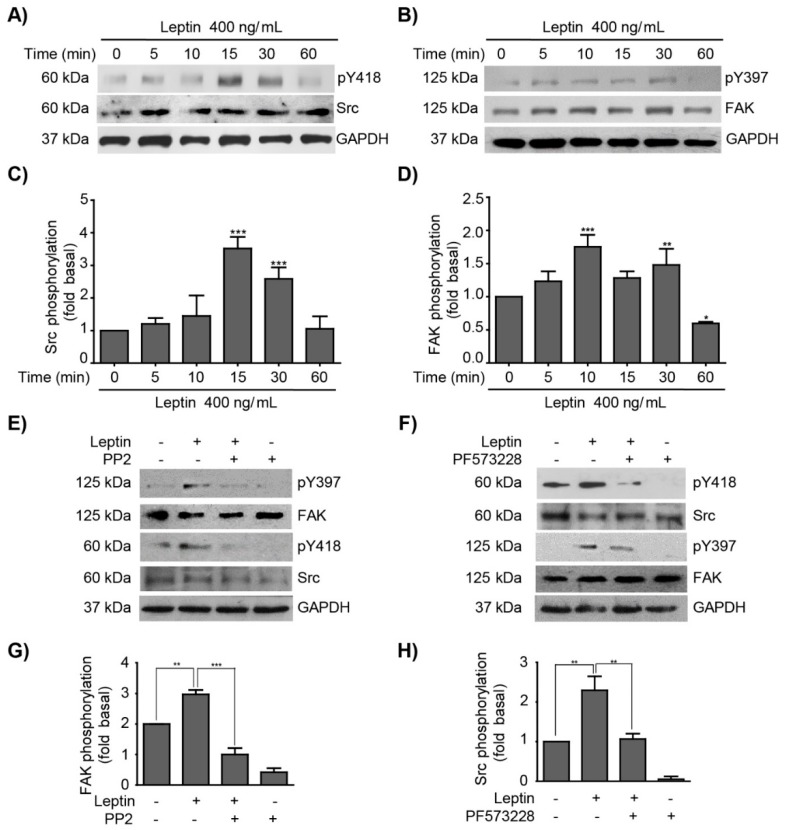
Figure 1.
Leptin activates Src and FAK in the non-tumorigenic breast epithelial cell line MCF10A. Representative Western blots of whole cell extracts obtained at different time points from MCF10A cells stimulated with 400 ng/mL of leptin. (A) Effect of leptin in Src activation was detected with an anti-p-Y418 antibody and was compared to total Src. (B) Effect of leptin in FAK activation was detected with an anti-p-Y397 antibody and was compared to total FAK. GAPDH was used as loading control. Densitometric analyses of (C) Src, and (D) FAK phosphorylation dependent on leptin. MCF10A cells were pre-treated with Src (PP2) or FAK (PF-573228) inhibitors, and subsequently with leptin 400 ng/mL. Representative western blots of Src phosphorylated in Y418 (E), and FAK phosphorylated in Y397 (F). Control blots showing loss of pY418-Src and pY397-FAK in the presence of inhibitors, PP2 and PF573228 are shown. GAPDH was used as a loading control. Densitometric analyses of leptin-dependent (G) FAK, and (H) Src phosphorylation in the presence of the inhibitors. The values are shown in means ± SD of three independent experiments and are expressed as changes with respect to the control (unstimulated cells). The asterisks indicate the comparison made with respect to the control. * p < 0.05, ** p < 0.01 and *** p < 0.001 by one-way ANOVA (Dunnett’s and Newman-Keuls’s test).