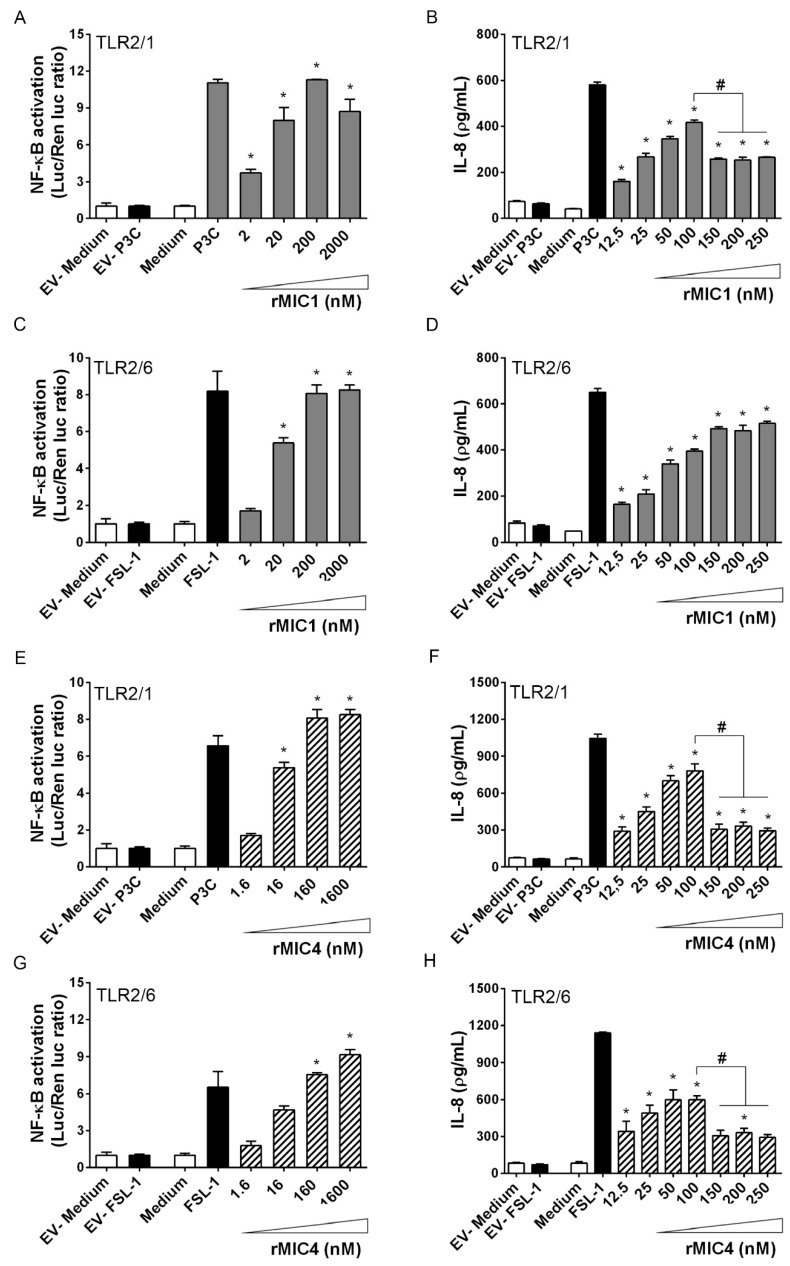
Figure 2.
TLR2 is required for in vitro cell activation induced by microneme proteins. HEK293T cells were transfected with CD14, CD36; a pair of TLRs (either TLR2/1 or TLR2/6); and an NF-κB-dependent luciferase reporter (pELAM-firefly luciferase) and a constitutive Renilla luciferase reporter construct (internal control). We maintained a constant amount of DNA in each transfection by adding an empty expression vector. After 48 h of transfection, we stimulated the cells with the following agonists: Pam3CSK4 (P3C, 1 nM) for cells transfected with TLR2/1 (A,B,E,F) and FSL-1 (1 nM) for cells transfected with TLR2/6 (C,D,G,H). Medium and an empty vector (stimulated with medium or an agonist) were used as negative controls for cell stimulation. Different concentrations of rMIC1 (A–D) or rMIC4 (E–H) (indicated on the coordinate axis) were used to stimulate the transfected cells. Cells were lysed at 4 h post-stimulation, and luminescence intensity was measured to assess NF-κB activation. The IL-8 concentration was measured in cell supernatants harvested at 24 h post-stimulation. The data are from three independent experiments yielding similar results. Statistical differences were determined by (*) comparing the responses of cells stimulated with microneme proteins to the responses of unstimulated cells (medium, negative control). In addition, statistical comparisons (#) of responses elicited by 100 nM of rMIC proteins versus the response in the 150, 200, and 250 nM of rMIC were also performed. (* and #) p < 0.05 by one-way ANOVA followed by Bonferroni’s post-test.