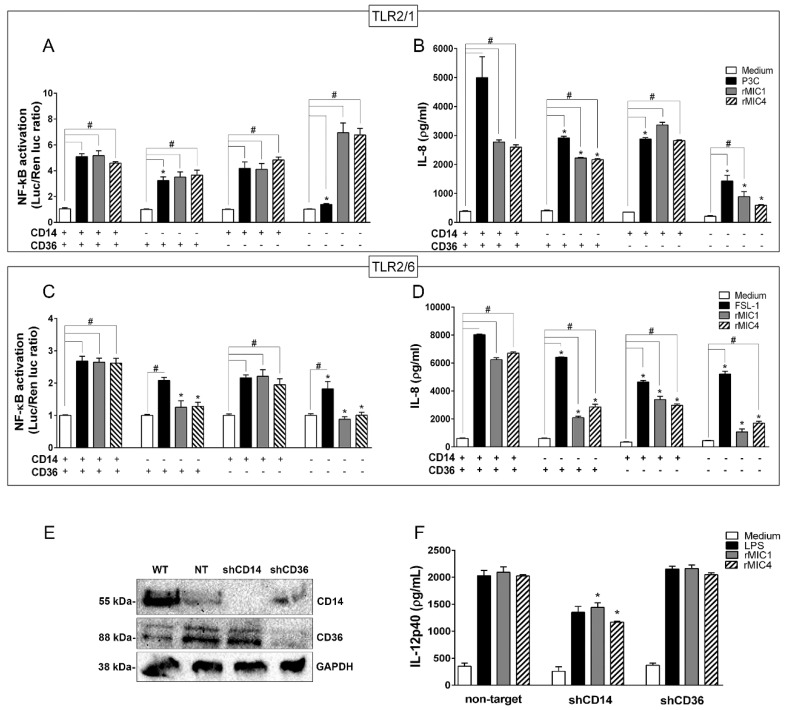
Figure 4.
The CD14 and CD36 co-receptors amplify TLR2-mediated cell activation induced by microneme proteins. HEK293T cells transfected with (A,B) TLR2/1 or (C,D) TLR2/6 were co-transfected with CD14 and CD36 either together or alone, along with an NF-κB-dependent luciferase reporter construct (pELAM-firefly luciferase) and a Renilla luciferase reporter construct (an internal control). The total amount of DNA in each transfection was kept constant by adding empty vector. After 48 h of transfection, the cells were stimulated with rMIC1 (50 nM) or rMIC4 (50 nM). Pam3CSK4 (P3C, 1 nM) was used as a positive control for TLR2/1, and FSL-1 (1 nM) was used as a positive control for TLR2/6. Medium was used as the negative control. Twenty-four hours post-stimulation, the IL-8 concentration in cell supernatants were assessed by ELISA. Statistical analysis was performed to compare (*) the responses in cells lacking one or more co-receptor to the responses when both CD14 and CD36 were expressed. The responses in cells expressing one co-receptor, both co-receptors, or no co-receptor (#) were compared after stimulation with microneme protein versus the responses in the negative control cells (medium). The data are from three independent experiments yielding similar results (E) BMDMs obtained from wild type C57BL/6 mice were transduced with lentivirus vectors encoding shRNA sequences for CD14 and CD36 or a non-target control shRNA. The expression levels of CD14 and CD36 were evaluated by immunoblotting. (F) BMDMs deficient in CD14 or CD36 were stimulated with rMIC1 (5 µg/mL) or rMIC4 (5 µg/mL). LPS (1 µg/mL) was used as a positive control, and medium was used as a negative control. The IL-12 concentration in cell supernatants was measured by ELISA. (*) Statistical analysis of the responses in CD14- or CD36-knockdown cells versus that in non-targeted shRNA-transduced cells. (*) or (#) p < 0.05 by one-way ANOVA. Data from two independent experiments yielding similar results.