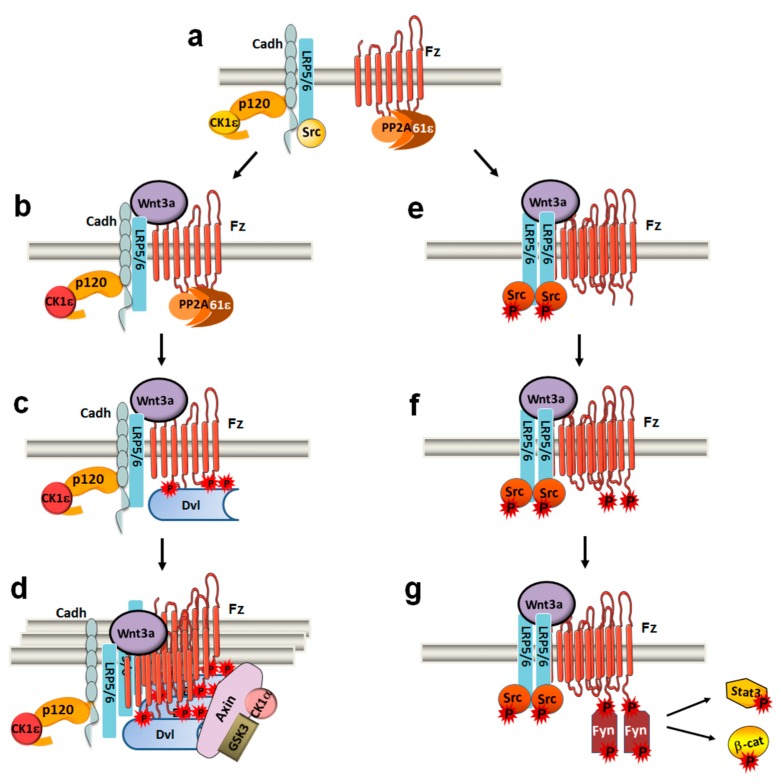
Figure 1.
Canonical Wnt ligands induce two mutually exclusive Dvl2- and Fyn-dependent arms. (a) In Wnt OFF, LRP5/6 co-receptor interacts through p120-catenin and E-cadherin with inactive CK1ε and Src (both inactive kinases are shown in yellow). (b) Wnt3a promotes that PP2A phosphatase, associated to Fz2 through the PR61ε regulatory subunit, moves closer to CK1ε, and dephosphorylates and activates CK1ε (in orange). (c) CK1ε increases Dvl2 phosphorylation and its binding to Fz2. (d) Dvl2′s association leads to signalosome assembly, axin recruitment, and further responses of this pathway. (e) LRP5/6 dimerization promotes Src activation and (f) Src-dependent phosphorylation of Tyr552 in Fz2. (g) Phospho-Tyr552 binds and activates Fyn, promoting the phosphorylation of Stat3. Fyn also phosphorylates β-catenin Tyr142, releasing β-catenin from α-catenin and cadherin and thereby facilitating its transcriptional activity.