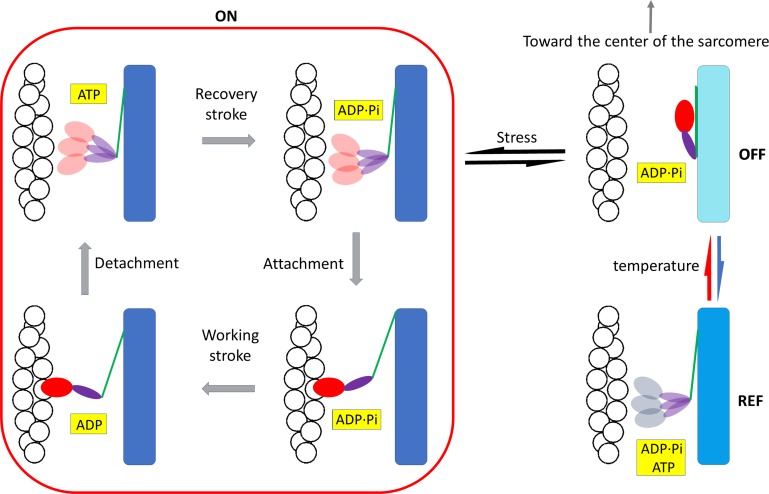
Figure 8.
Scheme of the regulatory effect of temperature on thick filament. Myosin motors at rest either lie on the surface of the thick filament in the ordered OFF state, folded back toward the center of the sarcomere, or are in a disordered state refractory to activation, REF. Lowering the temperature (blue arrow) favors the REF state, while increasing the temperature (red arrow) favors the OFF state. Upon muscle activation, only the fraction of motors in the OFF state are recruited, in relation to the stress on the thick filament (black arrows), into the disordered ON ADP.Pi-state, from which they can attach to actin and enter the mechanochemical cycle (bounded by the red line). The state of the ligand in the nucleotide-binding pocket of the motor is highlighted in yellow. The motor domain of the head is red except in the REF state (gray), for which the state of the ligand is not defined. The backbone of the thick filament has a shorter periodicity in the OFF state (cyan), which increases both in the REF state (light blue) or when the thick filament is switched ON (blue) because motors have moved away from the ordered helical disposition along the surface of the filament.