Figure 5.
ALDH1 Expression Is Associated with Advanced Clinical Stage, Poor Prognosis, and Paclitaxel Sensitivity in Human Endometrial Cancer
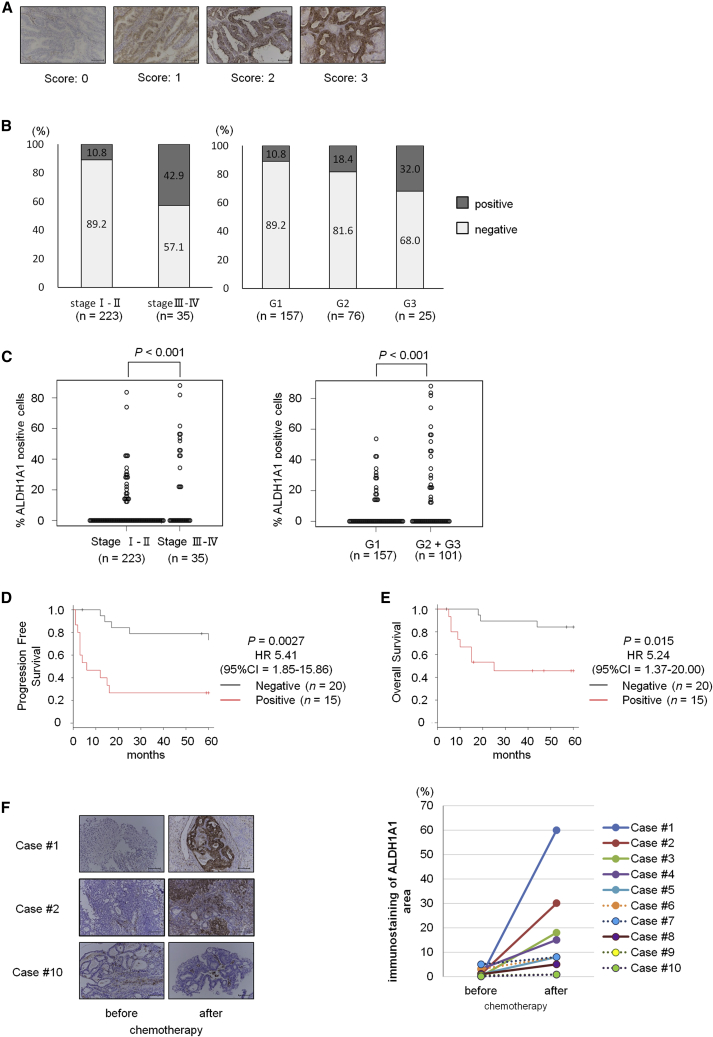
(A) Representative immunostaining of ALDH1A1 (score 0–3). Scale bars, 100 μm.
(B) Distribution of ALDH1A1 expression during different clinical stages in endometrial cancer, and distribution of ALDH1A1 expression in different histological grades of endometrial cancer (n = 258).
(C) Percentage of ALDH1A1-expressed cancer cells in different clinical stage or histological grades of endometrial cancer (n = 258).
(D and E) Kaplan-Meier analyses of progression-free survival (D) and overall survival (E) in patients with advanced-stage endometrial cancer. The patients were stratified into ALDH1A1-positive (red lines, n = 15) and ALDH1A1-negative (gray lines, n = 20) groups. Most of these patients were treated with taxane-containing chemotherapy as the first-line regimen.
(F) Immunostaining for ALDH1A1 in uterine tumors before and after chemotherapy. Scale bars, 100 μm (left). ALDH1A1 immunostaining area in uterine tumors before and after chemotherapy (right). Cases of ALDH1A1 staining area expanded up to five times are indicated as straight lines (n = 6), and other cases are indicated as dotted lines (n = 4).
See also Table S3.