Figure 4.
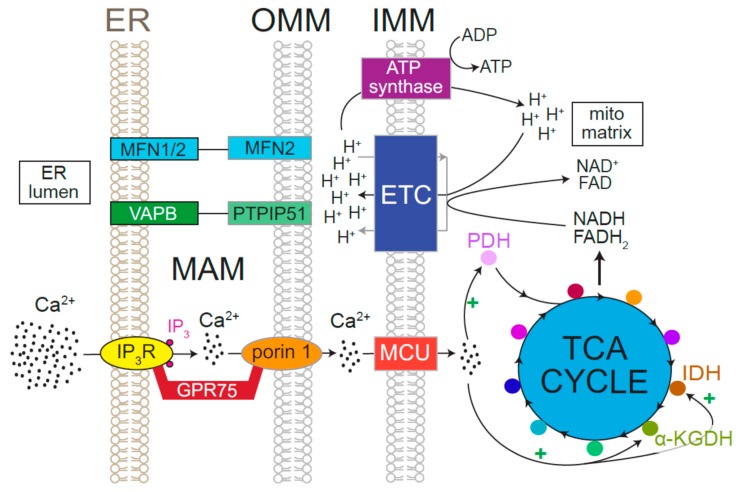
ER Ca2+ release stimulates oxidative phosphorylation. Mitochondria-associated membranes (MAMs) are points of contact between the ER and mitochondria that have various functions, including Ca2+ signaling between the two organelles. MAMs are stabilized by protein pairs that bind to each other, serving as molecular tethers. The VAPB-PTPIP51 and MFN1/2-MFN2 binding pairs are two such examples. IP3R exists in MAMs and is linked via GPR75 to porin 1, which is found in the outer mitochondrial membrane (OMM). In this arrangement, IP3-mediated Ca2+ release enables efficient transfer of Ca2+ into the intermembrane space, where the low-affinity mitochondrial Ca2+ uniporter (MCU) can be overcome by concentrated Ca2+ microdomains. Ca2+ that enters the mitochondrial matrix stimulates the tricarboxylic acid (TCA) cycle by activating pyruvate-dehydrogenase (PDH), isocitrate-dehydrogenase (IDH), and alpha-ketoglutarate dehydrogenase (KGDH). The TCA cycles produces the reducing equivalents (NADH and FADH2) that power the electron transport chain (ETC), which produces a protein gradient in the intermembrane space. Protons (H+) flow down their concentration gradient through ATP synthase leading to biogenesis of ATP.