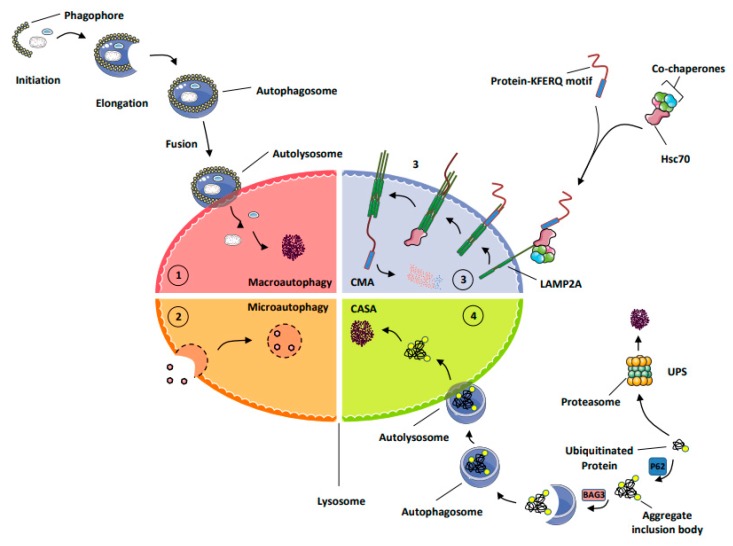
Figure 1.
Schematic representation of the main processes of protein degradation. (1) MA (macro-autophagy) triggers the degradation of proteins, protein aggregates, lipids, and carbohydrates but also damaged organelles, as well as intracellular micro-organisms into the lysosomes. (2) Micro-autophagy corresponds to a less-selective form of autophagy that is carried out through the invagination of the lysosomal membrane around the material to be degraded. (3) Chaperone-mediated autophagy (CMA) allows the degradation of cytosolic proteins endowed with a KFERQ-like motif. During CMA, this motif is recognized by Hsc70, also called HSPA8, which triggers their unfolding and subsequent transport into the lysosome, where they are ultimately processed by lysosomal proteases. (4) Chaperone-assisted selective autophagy (CASA) ensures the selective ubiquitin-dependent degradation of dysfunctional chaperone-bound proteins in lysosomes. The ubiquitin proteasome system (UPS) is the cellular process by which short-lived proteins and dysfunctional or unfolded proteins are addressed to the proteasome for degradation.