Abstract
Background
Better screening and testing approaches are needed to improve TB case finding, particularly in health facilities where many people with TB seek care but are not diagnosed using the existing approaches.
Objective
We aimed to evaluate the performance of various TB screening and testing approaches among hospital outpatients in a setting with a high prevalence of HIV/TB.
Methods
We screened outpatients at a large hospital in Cameroon using both chest X-ray and a symptom questionnaire including current cough, fever, night sweats and/or weight loss. Participants with a positive screen were tested for TB using smear microscopy, the Xpert MTB/RIF assay, and culture.
Results
Among 2051 people screened, 1137 (55%) reported one or more TB symptom and 389 (19%) had an abnormal chest X-ray. In total, 1255 people (61%) had a positive screen and 31 of those screened (1.5%) had bacteriologically confirmed TB. To detect TB, screening with cough >2 weeks had a sensitivity of 61% (95% CI, 44–78%). Screening for a combination of cough >2 -weeks and/or abnormal chest X-ray had a sensitivity of 81% (95% CI, 67–95%) and specificity of 71% (95% CI, 69–73%), while screening for a combination of cough >2 weeks or any of 2 or more symptoms had a similar performance. Smear microscopy and Xpert MTB/RIF detected 32% (10/31) and 55% (17/31), respectively, of people who had bacteriologically-confirmed TB.
Conclusions
Screening hospital outpatients for cough >2 weeks or for at least 2 of current cough, fever, night sweats or weight loss is a feasible strategy that had a high relative yield to detect bacteriologically-confirmed TB in this population. Clinical diagnosis of TB is still an important need, even where Xpert MTB/RIF testing is available.
Keywords: Tuberculosis, HIV/AIDS, Case detection, Molecular diagnostics, Chest X-ray
1. Introduction
Many people who have tuberculosis (TB) are never diagnosed or treated, resulting in high rates of mortality and ongoing transmission of the disease [1]. Globally, stagnating numbers of people diagnosed and notified as having TB has fueled an interest in studies that evaluate methods to improve case detection [2], [3], [4], [5], [6]. There is evidence that many people with TB present at health facilities, yet are not identified as needing evaluation for TB [7], [8], [9]. Some of these initially undiagnosed TB patients who attend health facilities either recover spontaneously or eventually develop more severe symptoms and are diagnosed, but autopsy studies indicate many others die without ever being diagnosed, especially in African settings with high HIV co-infection rates [10].
In many settings, screening for TB is performed by asking people if they have had cough more than two weeks, although this approach alone has been shown to have limited sensitivity to detect TB [11], [12]. Multi-symptom screening is more sensitive and is recommended particularly among people with HIV [13], [14], [15]. Following a positive screen, the most widely used laboratory method for diagnosis of TB is sputum-smear microscopy, which has a sensitivity of <50% and is less sensitive among people living with HIV [16]. The Xpert MTB/RIF assay is a rapid, PCR-based TB test that has been recommended by the WHO to be used instead of smear microscopy [17]; however, while usage is increasing, the assay is not yet widely used in many high burden countries [18]. After a lab test where TB is not detected, the WHO recommends that someone under evaluation for TB should receive a chest X-ray. If the chest X-ray is suggestive of TB and the person does not respond to broad spectrum antibiotics or other appropriate treatment, then they may be diagnosed on clinical grounds without microbiological confirmation and treated for TB [19], [20]. In practice, however, multiple visits incur costs for patients, and many people drop out along the diagnostic pathway.
For most of the last three decades, the widespread use of chest X-ray in TB programs has been discouraged due to its unreliability for TB diagnosis [21], [22]. However, recently more attention is being given to the role of chest X-ray to aid in the detection of TB, with a focus on using chest X-ray as a screening tool to complement or replace symptom screening or as a triage tool to increase the efficiency of Xpert MTB/RIF testing [23], [24], [25], [26]. Although screening by chest X-ray has been shown to be sensitive for identifying people with TB in prevalence surveys and through modeling [12], [27], this approach has not been widely adopted or tested in different resource-limited settings, often due to one or more issues including cost, access, or shortage of qualified readers [28].
In this study, we aimed to assess the TB burden and the yield of various enhanced TB screening strategies to detect TB among hospital outpatients. We used an approach similar to community TB prevalence surveys. [29] People presenting to the hospital outpatient department received a parallel multi-symptom screen and chest X-ray screen, and anyone with either an abnormal chest X-ray or any TB symptom was referred for TB testing using smear microscopy, the Xpert MTB/RIF assay and culture. This was performed in Cameroon, a high TB/HIV burden country where 4.3% of adults are HIV-positive, the estimated TB incidence is 203/100,000, and 34% of people notified with TB are co-infected with HIV. In this setting, nearly all TB diagnostic testing is performed using TB microscopy, and fewer than 10% of people evaluated in routine testing for TB receive an Xpert MTB/RIF test or TB culture. An estimated 48% of incident TB cases are missed by the Cameroon National TB Program (NTP)[1], so identifying better ways to screen people for TB in healthcare facilities could facilitate approaches to improve TB treatment coverage in this setting.
2. Methods
2.1. Setting and participants
This cross-sectional study was conducted at the Tuberculosis Reference Laboratory Bamenda and the Bamenda Regional Hospital as part of a TB REACH project. The Bamenda Regional Hospital receives patients from Bamenda, the regional capital, as well as patients referred from around Cameroon's Northwest region (population ∼1.95 million). The imaging center of the hospital has a digital radiography system (Carestream Direct View Classic CR). The Tuberculosis Reference Laboratory Bamenda is a regional reference laboratory of the National TB Program that serves four of the ten regions of the country; the facility is accredited in accordance with the recognized International Standard ISO 15189:2012 (SANAS Accredited Medical Laboratory, No. M0593). This study was approved by the National Ethics Committee of Cameroon.
Each morning, consecutive adults presenting at the outpatient department of the Bamenda Regional Hospital were screened for eligibility and invited to participate in the study. According to the client flow at the hospital, people presenting for consultation first pass through this outpatient department and are then referred as needed for other services, such as diagnostic testing or consultation with a specialist. At the hospital, there is a separate HIV treatment center, so people under care for HIV may go either directly to the HIV treatment center or to the outpatient department for consultation. Because the study was performed in parallel with routine hospital activities, the capacity of the imaging center typically allowed for 20–25 people per day being invited to participate, representing approximately 40% of the hospital outpatients; when this number was reached, enrollment was halted until the following day. We aimed to invite 3000 people to participate, which was the number feasible for us to enroll with the resources available during eight months, and we estimated that 45–50% of participants would have a positive screen and be sent for laboratory testing. All those ≥15 years of age were eligible. People were excluded if they were already on anti-TB treatment or if they were possibly or known to be pregnant, in order to minimize potential fetal exposure to X-rays following the hospital practice. People were recruited regardless of the presence or absence of symptoms or clinical suspicion of TB.
After providing written informed consent, participants completed a standardized interview including the TB symptom screening questions of whether or not they had cough of any duration, fever, night sweats and weight loss [14]. Participants also received a free onsite posterior-anterior digital chest X-ray read by a trained radiologist who did not have access to the symptom screening results or other clinical information. The chest X-ray was classified as normal or abnormal using the TB prevalence survey guidelines to screen for any abnormality [29], and this was used as the chest X-ray screening result. Anyone with one or more symptoms and/or a chest X-ray with any abnormality was referred for TB laboratory testing on the same day.
2.2. Laboratory methods
People presenting at the laboratory were instructed on how to produce two sputum specimens, one spot and one early morning. Smears were prepared directly from the first sputum collected (typically spot) and examined for acid-fast bacilli by fluorescence microscopy. The second sputum specimen (typically morning) was processed using the N-acetyl-L-cysteine-NaOH (NALC—NaOH) method [30]. From the re-suspended pellet, a concentrated smear was prepared and examined for acid-fast bacilli by fluorescence microscopy, 0.5 mL was used for Xpert MTB/RIF assay (Cepheid, Sunnyvale USA), 0.5 mL was inoculated on mycobacterial growth indicator tubes (MGIT, BD Diagnostic Systems, Sparks, MD, USA) using the BACTEC MGIT 960 system, and ∼0.1 mL was inoculated on Löwenstein-Jensen media. Cultures positive for acid-fast bacilli were tested for M. tuberculosis complex by MPT64 antigen detection (Standard Diagnostics, Republic of Korea). Isolates that were AFB-positive and negative by MPT64 antigen testing were tested using the Genotype Mycobacterium CM line probe assay (Hain Lifescience, Germany) according to the manufacturer's instructions. Cultures were read without reference to any prior diagnostic test results. To assess whether positive cultures may have been the result of cross-contamination during specimen processing, all positive cultures within each processing batch (from both study and non-study patients) were sent to ITM Antwerp for comparison by spoligotyping and mycobacterial interspersed repetitive-unit variable-number tandem repeat (MIRU-VNTR) analysis. People presenting at the laboratory for TB testing were offered a free HIV test as part of the study. Anyone with bacteriologically-confirmed TB who had not previously received an HIV test was followed up and offered a free HIV test following the NTP protocol for newly diagnosed TB patients.
2.3. Data analysis
Participants with one or more Xpert MTB/RIF result and/or sputum culture positive for M. tuberculosis complex were defined as having bacteriologically confirmed tuberculosis. Microscopy smears were graded as positive (3+, 2+, 1+ or scanty) or negative. The Xpert MTB/RIF assay reports a semi-quantitative grade for positive results based on bacterial load (high, medium, low, very low). The automated liquid culture (BD MGIT 960) instrument records the time to detection (TTD) of culture positivity in hours; this variable corresponds to the number of colony-forming units (CFU) per milliliter and therefore provides a measure of the density of viable bacilli in the sputum [31].
Data were entered and analyzed using EpiData (www.epidata.dk). Data from the study files and laboratory registers were double entered and validated by comparison. Participants were characterized using simple descriptive statistics. Proportions were compared using the χ2-test or Fisher's exact test as appropriate. All statistical tests were 2-sided at α = 0.05. The sensitivity and specificity of different screening approaches to detect bacteriologically-confirmed TB were determined using the total number of participants with complete screening results as the denominator, although not all the participants received the reference standard laboratory tests. This approach therefore assumes that both people with a negative screen (no TB symptoms and normal chest X-ray) and people with a positive screen who did not provide a specimen for lab testing did not have bacteriologically-confirmed TB; with this approach, the TB burden is likely somewhat underestimated and the sensitivity of various screening approaches is likely somewhat overestimated. We performed the analysis in this way to facilitate comparison between different screening approaches and to enable comparison to other studies that used the same assumptions [5], [12]. The STROBE recommendations were followed for reporting these results [32].
3. Results
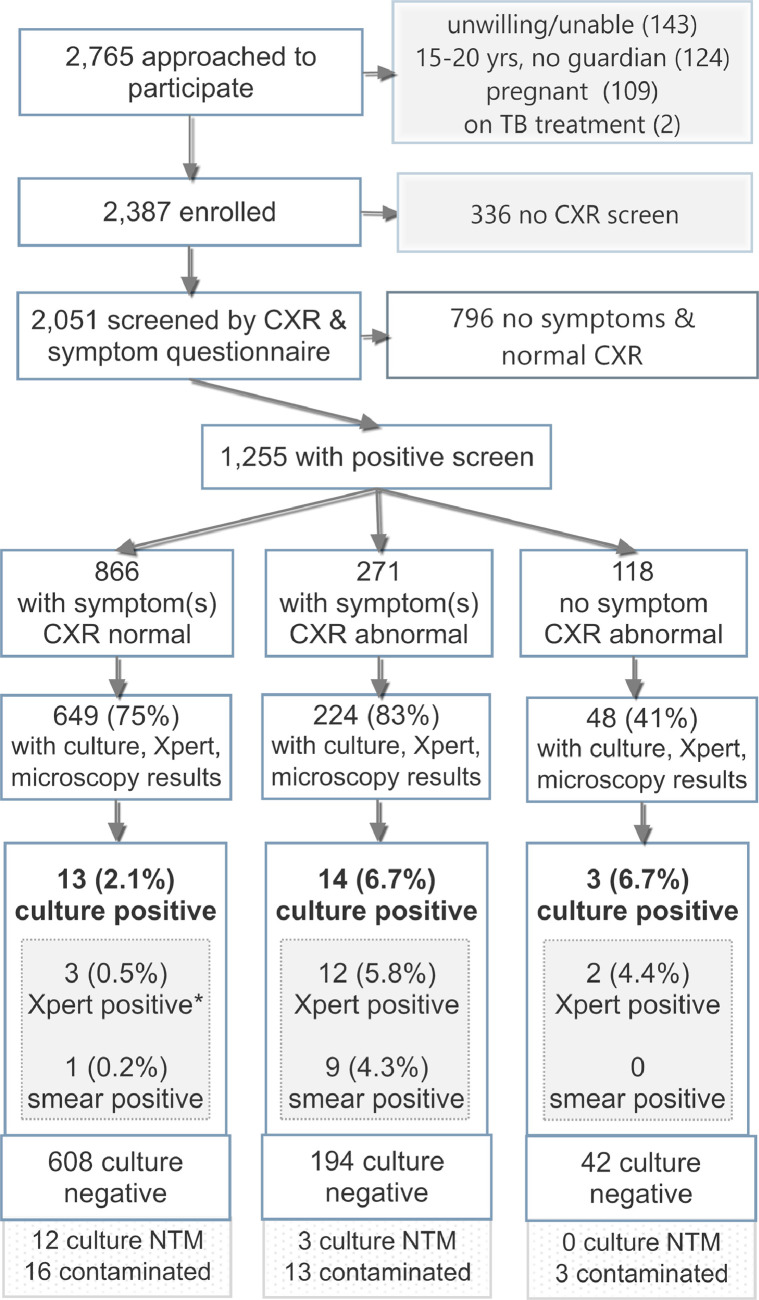
Between September 8, 2015 and April 15, 2016, 2765 hospital outpatients were invited to participate in the study and 2387 were enrolled (Fig. 1). Of these, 2051 participants (86%) were screened both for TB symptoms and by chest X-ray; the other 336 people were screened for symptoms but did not receive a chest X-ray after they had been recruited due to time and capacity restrictions in the imaging center, primarily due to use of the instrument for more urgent cases on some days.
Fig. 1.
Flow diagram of the study population with screening and specimen laboratory testing results.
*One of the 17 people with positive Xpert results had a negative culture result; the other 16 had culture results positive for TB. CXR, chest X-ray; NTM, non-tuberculous mycobacteria; TB, tuberculosis Xpert, Xpert MTB/RIF assay.
Among the 2051 people with both symptom and chest X-ray screening results (Table 1), the median age was 39 (IQR 28–55), 1279 (62%) were female, 55 (2.7%) had a history of TB treatment. Overall, 389 (17%) had an abnormal chest X-ray, 1137 (55%) reported one or more TB symptom, 867 people (42%) reported current cough, and 341 people (17%) reported cough greater than 2 weeks. Those with an abnormal chest X-ray were older than those with a normal chest X-ray (p < 0.001), and those reporting any of the 4 symptoms were older than those reporting no symptoms (p < 0.001).
Table 1.
The characteristics of 2051 hospital outpatients screened for TB, according to chest X-ray result and symptoms reported.
| All | Chest X-ray screening |
Symptom screening |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Normal |
Abnormal |
No symptom |
Any symptom(s) |
|||||||
| Characteristics | n = 2051 | n = 1662 | n = 389 | n = 914 | n = 1137 | |||||
| Age, years, median (IQR) | 39 | (28–55) | 36 | (27–51) | 60 | (42–70) | 36 | (27–48) | 44 | (30–60) |
| Sex, Female, n (%) | 1279 | (62) | 1038 | (63) | 241 | (62) | 567 | (62) | 712 | (63) |
| Male, n (%) | 772 | (37) | 624 | (38) | 148 | (38) | 347 | (38) | 425 | (37) |
| No prior TB treatment,*n (%) | 1982 | (97) | 1616 | (98) | 366 | (96) | 891 | (98) | 1091 | (97) |
| Prior TB treatment, n (%) | 55 | (3) | 38 | (2) | 17 | (4) | 20 | (2) | 35 | (3) |
14 people did not have a recorded answer for whether they had previously taken TB treatment.
Complete TB lab testing results were available for 872 (69%) of those with a positive screen; 334 people eligible for sputum collection either did not go to the laboratory or did not produce a sputum specimen despite coaching, and 47 had incomplete or non-interpretable TB lab results; as shown in the Fig. 1, these include those with culture results of non-tuberculosis mycobacteria (NTM, 15/921 = 1.6%) or contaminated culture results (32/921 = 3.5%).
Of the 31 people with bacteriologically confirmed TB, 10 (32%) were positive by concentrated smear microscopy, 17 (55%) were positive on the Xpert MTB/RIF assay and 30 (97%) were positive by TB culture. Among those with culture-positive TB, 17 (57%) had a positive culture on solid media and 29 (97%) had positive culture on liquid media (MGIT). Of the 16 people with Xpert-positive and culture-positive results, 2 had Xpert results with grade high, 5 with medium, 5 low and 4 very low. Two patients with specimens positive for Mycobacterium tuberculosis complex had specimens with resistance to rifampin; one of these patients was started on MDR-TB treatment, while the other died two days after the diagnosis without starting treatment. Only one person who tested positive on the Xpert MTB/RIF assay (with a grade of very low) had a negative culture result; this person had no history of TB treatment, reported all four symptoms, and had a normal chest X-ray. Two participants had sputum-smear positive (scanty) results and negative Xpert and culture results; both of these participants had symptoms of TB and were reported as AFB smear-positive for TB following national guidelines but were not considered confirmed TB cases for this study. In addition to the 30 cultures positive for M. tuberculosis complex, there were 15 specimens with smear-negative microscopy results and cultures positive for nontuberculous mycobacteria (including 5 M. fortuitum, 2 M. gordonae, 3 M. intracellulare, and 5 non-speciated mycobacteria).
Among the 2051 people screened, 31 had bacteriologically-confirmed TB (1.5%, 95%CI 1.1–2.1%, Table 2). For people reporting cough >2 weeks, 5.6% (95% CI, 3.6–8.5%) had bacteriologically-confirmed TB. By number of symptoms reported, the proportion with TB varied from 0.3% among those reporting none of the 4 symptoms up to 8.3% among those reporting all 4 symptoms (p < 0.001). While 4.4% of the participants with an abnormal chest X-ray had TB, only 0.8% of those with a normal chest X-ray had confirmed TB (p < 0.001).
Table 2.
The characteristics of 2051 people screened for TB according to whether TB was confirmed, and the number needed to screen (NNS) to identify one person with TB.
| Characteristic | All (n) | TB confirmed (n) | TB confirmed (%, 95% CI) | p-value | Number needed to screen, NNS | ||
|---|---|---|---|---|---|---|---|
| Total | 2051 | 31 | 1.5% | (1.1–2.1) | 66 | ||
| Age (years) | 15–34 | 820 | 13 | 1.6% | (0.9–2.7) | 0.32 | 63 |
| 35–54 | 670 | 13 | 1.9% | (1.1–3.3) | 52 | ||
| 55+ | 561 | 5 | 0.9% | (0.4–2.1) | 112 | ||
| Sex | Male | 772 | 15 | 1.9% | (1.2–0.3.2) | 0.26 | 51 |
| Female | 1279 | 16 | 1.3% | (0.8–2.0) | 80 | ||
| Prior TB treatment (n = 2037) | No | 1982 | 30 | 1.5% | (1.1–2.2) | 0.57 | 66 |
| Yes | 55 | 1 | 1.8% | (0.3–9.6) | 55 | ||
| Number of symptoms (CFSW) | 0 | 914 | 3 | 0.3% | (0.1–1.0) | <0.001 | 305 |
| 1 | 500 | 4 | 0.8% | (0.3–2.0) | 125 | ||
| 2 | 473 | 16 | 3.4% | (2.1–5.4) | 30 | ||
| 3 | 116 | 4 | 3.4% | (1.3–8.5) | 29 | ||
| 4 | 48 | 4 | 8.3% | (3.3–19.6) | 12 | ||
| Current cough* | No | 1184 | 4 | 0.3% | (0.1–0.9) | <0.001 | 296 |
| Yes | 867 | 27 | 3.1% | (2.1–4.5) | 32 | ||
| Cough >2 weeks | No | 1710 | 12 | 0.7% | (0.4–1.2) | <0.001 | 143 |
| Yes | 341 | 19 | 5.6% | (3.6–8.5) | 18 | ||
| Fever | No | 1724 | 20 | 1.2% | (0.8–1.8) | 0.006 | 86 |
| Yes | 327 | 11 | 3.4% | (1.9–5.9) | 30 | ||
| Night sweats | No | 1791 | 19 | 1.1% | (0.7–1.7) | <0.001 | 94 |
| Yes | 260 | 12 | 4.6% | (2.7–7.9) | 22 | ||
| Weight loss | No | 1472 | 14 | 1.0% | (0.6–1.6) | 0.002 | 105 |
| Yes | 579 | 17 | 2.9% | (1.8–4.7) | 34 | ||
| Chest x-ray abnormal by radiologist | No | 1662 | 14 | 0.8% | (0.5–1.4) | <0.001 | 119 |
| Yes | 389 | 17 | 4.4% | (2.7–6.9) | 23 | ||
| HIV-positive (30 TB cases) | No | 20 | |||||
| Yes | 10 | ||||||
CFSW: C, current cough, F, fever, S, night sweats, W, weight loss.
Of those with bacteriologically confirmed TB, 30 (97%) were tested for HIV and 10 people (33%) were HIV-positive (Table 2). Among those presenting at the laboratory who did not subsequently have bacteriologically confirmed TB, 32% (271/841) consented to be tested for HIV and 7% (18 people) were HIV-positive; the age and sex distribution of those tested and those not tested for HIV was similar.
We compared the sensitivity and specificity of various screening approaches and combinations of screening approaches (Table 3). The sensitivity of screening for cough >2 weeks alone was 61% (95% CI, 44–78%), with a specificity of 84% (95% CI, 82–86%), and the sensitivity of abnormal chest X-ray alone was 55% (37–72%) with a specificity of 82% (80–83%). Combining either cough >2 weeks and/or abnormal chest X-ray had a sensitivity of 81% (67–95%) with a specificity of 71% (95% CI, 69–73%). Screening for at least 2 symptoms had a sensitivity of 77% (95% CI, 63–92%), while combining either cough >2 weeks and/or at least 2 symptoms increased the sensitivity to 87% (95% CI, 75–99%), with specificity decreasing to 63% (61–65%).
Table 3.
The performance of various combinations of screening approaches to detect TB among hospital outpatients.
| Screening approach | Sensitivity (95%CI) | Specificity (95% CI) | ||
|---|---|---|---|---|
| Abnormal chest X-ray OR any symptom* | 100 | – | 39 | (37–42) |
| Abnormal chest X-ray OR cough >2 weeks | 81 | (67–95) | 71 | (69–73) |
| Abnormal chest X-ray | 55 | (37–72) | 82 | (80–83) |
| Current cough | 87 | (75–99) | 58 | (56–61) |
| Cough >2 weeks | 61 | (44–78) | 84 | (82–86) |
| Fever | 35 | (19–52) | 84 | (83–86) |
| Night sweats | 39 | (22–56) | 88 | (86–89) |
| Weight loss | 55 | (37–72) | 72 | (70–74) |
| At least 1 symptom(s) | 90 | (80–100) | 45 | (43–47) |
| At least 2 symptoms | 77 | (63–92) | 70 | (68–72) |
| At least 3 symptoms | 26 | (10–41) | 92 | (91–93) |
| All 4 symptoms | 13 | (1–25) | 98 | (97–98) |
| Cough > 2 weeks and/or at least 2 symptoms | 87 | (75–99) | 63 | (61–65) |
| Cough > 2 weeks and/or at least 3 symptoms | 68 | (51–84) | 80 | (78–81) |
Only those people who reported any of current cough, night sweats, weight loss or fever or who had an abnormal chest X-ray were referred for TB lab testing.
Among the 14 people with culture-positive TB and an Xpert-negative result, 13 (93%) reported current cough and 7 (50%) reported cough more than two weeks (Table 4). As compared to those with Xpert-positive TB, the people with Xpert-negative, culture-positive TB were older, more likely to be female, and more likely to have a normal chest X-ray, while the prevalence of HIV was similar in the two groups. The median time to detection on culture was significantly longer for those with Xpert-negative/culture-positive TB.
Table 4.
Characteristics of 31 patients with bacteriologically confirmed TB stratified by whether TB was detected using Xpert MTB/RIF assay.
| Bacteriologically-confirmed TB (n = 31) |
|||
|---|---|---|---|
| Characteristic | Xpert-negative (n = 14) | Xpert-positive (n = 17) | p-value |
| Age (years), median (IQR) | 52 (43–65) | 31 (24–38) | <0.001 |
| Female | 11 (79) | 5 (29) | 0.01 |
| Prior TB treatment | 1 (7) | 0 (0) | 0.45 |
| Any TB symptom* | 13 (93) | 15 (88) | 1 |
| Current cough | 13 (93) | 14 (82) | 0.61 |
| Cough >2 weeks | 7 (50) | 12 (71) | 0.28 |
| Fever | 3 (21) | 8 (47) | 0.26 |
| Night sweats | 4 (29) | 8 (47) | 0.46 |
| Weight loss | 5 (36) | 12 (71) | 0.08 |
| HIV-positive | 4 (29) | 6 (38) | 0.71 |
| CXR abnormal | 3 (21) | 14 (82) | 0.001 |
| Time to detection on culture (hours), median (IQR), n = 23 | 464 (170–860) | 144 (114–304) | 0.02 |
Any TB symptom refers to the WHO-recommended four-symptom screen for current cough, fever, night sweats and/or weight loss; CXR, chest X-ray; Xpert, Xpert MTB/RIF assay.
Of the 30 culture-positive specimens, cross contamination was excluded as a possibility for 27 of these, as 12 (40%) were the only specimens with positive culture results in their batch, 8 (27%) were in batches where none of the other culture positives in the batch had matching spoligotype results, and 7 (23%) were in batches with one or more matching spoligotype result, but those with matching spoligotype results had distinct MIRU-VNTR patterns. Of the remaining 3 people (10%) with positive cultures, cross-contamination is possible but unlikely, based on both the specimen position in the batch (not adjacent to specimens with matching MIRU-VNTR results) and due to low bacterial load (adjacent specimens were smear-negative/culture-positive).
4. Discussion
We assessed the performance of different combinations of screening with symptoms and chest X-ray to detect TB among outpatients in a large hospital in Cameroon. We found that the approach of screening for TB combining cough ≥2 weeks and/or chest X-ray had a similar sensitivity and only slightly higher specificity as combined screening with cough ≥2 weeks or at least 2 or more symptoms of cough, fever, night sweats or weight loss. Since chest X-ray is not available or feasible as a mass screening tool in most health facilities in Cameroon, an alternative symptom-only screening approach may be a good strategy to improve the detection of TB among people attending health facilities in this high HIV/TB burden setting.
The sensitivity of chest X-ray screening to detect bacteriologically-confirmed TB in this study (55%, 95% CI 37–72%) was lower than reported for community-based TB prevalence studies, where chest X-ray sensitivity has typically been reported to be higher than 90% [27], [33], [34], [35], [36]. The sensitivity of chest X-ray for TB is likely to vary both by setting and population screened, and there is little known about the sensitivity of chest X-ray for TB screening in health facilities. In a recent meta-analysis, only one study was included that had used chest X-ray for screening in a health facility, with a reported sensitivity of 26% (95% CI, 13–44%) and specificity of 92% (95% CI, 83–97%) [12]. Differences in the performance of chest X-ray in people attending healthcare facilities as compared to the community may reflect the different care seeking behavior of these two groups, or other factors. More information is needed about the performance of chest X-ray for TB screening in outpatients in other settings to develop a better understanding of its general usefulness in this population. In addition to performance, cost and access issues are also important considerations when evaluating whether to use chest X-ray as a screening tool in different settings [37].
A number of implementation studies evaluating Xpert MTB/RIF have shown that using it in lieu of or following smear microscopy can improve yields by 50–100% [38], [39]. The same was found in our study, but adding culture to the algorithm provided evidence that many more people with TB are still missed by the Xpert MTB/RIF assay. The sensitivity of Xpert in this population was lower than reported from a meta-analysis, but it was similar to that reported for a single MTB/RIF assay in other enhanced or active case finding studies [40], [41], [42], [43]. Those with Xpert-negative/culture-positive results had longer time to culture detection, reflecting lower bacilli density than those with Xpert-positive results, and these people were also much less likely to have an abnormal chest X-ray, as shown in Table 4. In Cameroon, culture is not recommended by the National TB Program for routine diagnosis of TB, due to the relatively high cost, limited facilities and slow turnaround time. It is not yet clear whether next generation molecular assays, such as the Xpert MTB/RIF Ultra [44], will be able to detect TB in these cases.
Using the approach described here, 1.5% of those screened and 3.6% of people tested had bacteriologically confirmed TB, including 1.1% with smear-positive TB. In Cameroon in 2016, 17% of those tested by smear microscopy in the country were smear-positive (NTP data). Our results suggest that enhanced screening could help to identify significantly more outpatients with TB, and much more can be done to identify people with suspected TB and ensure they complete the diagnostic pathway, despite the resulting increases in testing volumes and decreasing yields. Under the study conditions described here, people to be evaluated for TB benefited from careful symptom screening and follow-up according to the study testing algorithm; it is likely that better implementation of symptom screening, emphasis on good quality sputum production, and follow-up of all patients according to the recommended algorithms could contribute to better diagnoses under routine conditions as well.
It is also of note that 71% of pulmonary TB notifications are bacteriologically confirmed in Cameroon [1]. In practice, >90% of these bacteriologically-confirmed TB cases are smear-positive, since Xpert testing is not yet widespread. Our results indicate that there may be twice as many people with laboratory-confirmed, smear-negative disease who could be diagnosed either with more sensitive diagnostic tests or clinically, and that opportunities for treatment are routinely being missed due to an overreliance on smear-positive or even Xpert-positive results for TB diagnosis. While clinical diagnosis is a critical component for effective TB management, previous work indicates that clinical diagnosis is widely underutilized in this setting [45]. Since the conclusion of the study, several regions of the country have begun to implement a TB diagnostic algorithm that includes Xpert testing for priority populations, including people living with HIV, and follow-up after lab testing to improve the clinical diagnosis of TB.
In the region of the country where this study was conducted, 52% of the people notified as TB cases in 2016 were HIV-positive. The detection of a significantly lower proportion of HIV-positive TB cases in this study (33%, 10/30) as compared to routine practice suggests that more emphasis could be placed on TB screening in people with a negative or unknown HIV status. As has been shown in other settings, HIV-negative TB is an important driver of ongoing transmission, even in settings with a high prevalence of HIV/TB [46], [47], [48].
This study had several limitations. It is likely that the prevalence of TB among those screened is underestimated as a result of multiple factors. A significant proportion (27%) of people who screened positive (chest X-ray abnormal and/or any symptom) did not have a lab result, either because they did not go to the lab or were unable to produce sputum. Also, we did not systematically perform lab testing for participants without a positive symptom or chest X-ray screen, primarily due to cost and laboratory resource implications. Doing so would have allowed for a more complete sensitivity and specificity calculation and may have resulted in a slightly higher estimate of TB prevalence, although most data have shown little if any yield from people without symptoms and normal chest X-ray [29]. Only a single specimen from each participant was tested by Xpert and culture, while two specimens are recommended as the standard for culture and likely would have improved the overall yield. Due to logistical constraints for chest X-ray screening, we included only the first 20–25 people presenting at the outpatient department each day, or approximately 40% of all outpatients, and these patients may not be representative of all of those attending the outpatient department. In addition, since the chest X-ray reading was performed by a human reader, the results are subject to inter-reader variability, which is a well-known limitation for using chest X-ray as a screening tool. Early research on automated reading of chest X-ray films has shown that there is promising software to help standardize reading of images to help decide who needs further diagnostic testing, although its use must be evaluated in more settings [23].
5. Conclusions
Current facility-based TB case detection strategies in Cameroon and similar settings likely fail to detect many people with bacteriologically confirmed TB due to a combination of low rates of screening people attending the facilities, insensitive screening algorithms, inadequate performance of currently available laboratory tests, and/or a lack of clinical diagnosis. Our results suggest that enhanced screening for TB among hospital outpatients, for example by using a combination of either cough >2 weeks and/or at least 2 symptoms to identify those to receive a TB test, has the potential to enable a significant improvement in TB detection at healthcare facilities in this setting. Our results also suggest that enhanced TB screening needs to be implemented with the best diagnostic test available and combined with clinical diagnoses where needed, as the currently available molecular tests still miss many people with TB.
Acknowledgments
Acknowledgments
We thank the members of the team who contributed to patient care and data collection and those who performed laboratory testing, including Franklyn Nkongho and Comfort Vuchas. We also thank the people who participated in the study.
Authors' contributions
JC, AJC, CT, SL and MS designed the study. SL, CT, CL, and MS coordinated the study and the data collection at the site. MS, JC, and AJC, analyzed the data and wrote the first draft. All authors contributed to interpretation of data and revision of the manuscript, and all approved the final version before submission.
Funding
This work was supported by a grant from the Stop TB Partnership's TB REACH initiative using funds from Global Affairs Canada, and the National TB Program of Cameroon. BdJ was supported by an ERC-INTERRUPTB starting grant (nr. 311725).
Competing interests
AJC and JC work at the Stop TB Partnership. They do not participate in the funding decisions but provide technical support to projects once funded and approved.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jctube.2019.100095.
Appendix. Supplementary materials
References
- 1.World Health Organization . 2018. Global tuberculosis report 2018.https://apps.who.int/iris/handle/10665/274453 Geneva. [Google Scholar]
- 2.Corbett E.L., Bandason T., Duong T. Comparison of two active case-finding strategies for community-based diagnosis of symptomatic smear-positive tuberculosis and control of infectious tuberculosis in Harare, Zimbabwe (DETECTB): a cluster-randomised trial. Lancet. 2010;376:1244–1253. doi: 10.1016/S0140-6736(10)61425-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Golub J.E., Dowdy D.W. Screening for active tuberculosis : methodological challenges in implementation and evaluation. Int J Tuberc Lung Dis. 2013;17:856–865. doi: 10.5588/ijtld.13.0059. [DOI] [PubMed] [Google Scholar]
- 4.Creswell J., Sahu S., Blok L. A multi-site evaluation of innovative approaches to increase tuberculosis case notification: summary results. PLoS One. 2014;9:e94465. doi: 10.1371/journal.pone.0094465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van't Hoog A.H., Onozaki I., Lonnroth K. Choosing algorithms for TB screening: a modelling study to compare yield, predictive value and diagnostic burden. BMC Infect Dis. 2014;14:532. doi: 10.1186/1471-2334-14-532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Field S.K., Escalante P., Fisher D.A. Cough due to TB and other chronic infections: CHEST guideline and expert panel report. Chest. 2018;153:467–497. doi: 10.1016/j.chest.2017.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Claassens M.M., Jacobs E., Cyster E. Tuberculosis cases missed in primary health care facilities: should we redefine case finding? Int J Tuberc Lung Dis. 2013;17:608–614. doi: 10.5588/ijtld.12.0506. [DOI] [PubMed] [Google Scholar]
- 8.Chihota V.N., Ginindza S., Mccarthy K. Missed opportunities for TB investigation in primary care clinics in South Africa : experience from the XTEND trial. PLoS One. 2015;10 doi: 10.1371/journal.pone.0138149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O'Grady J., Bates M., Chilukutu L. Evaluation of the Xpert MTB/RIF assay at a tertiary care referral hospital in a setting where tuberculosis and HIV infection are highly endemic. Clin Infect Dis. 2012;55:1171–1178. doi: 10.1093/cid/cis631. [DOI] [PubMed] [Google Scholar]
- 10.Bates M., Mudenda V., Shibemba A. Burden of tuberculosis at post mortem in inpatients at a tertiary referral centre in sub-Saharan Africa: a prospective descriptive autopsy study. Lancet Infect Dis. 2015;15:544–551. doi: 10.1016/S1473-3099(15)70058-7. [DOI] [PubMed] [Google Scholar]
- 11.van't Hoog A.H., Onozaki I., Lonnroth K. Choosing algorithms for TB screening: a modelling study to compare yield, predictive value and diagnostic burden. BMC Infect Dis. 2014;14:1–12. doi: 10.1186/1471-2334-14-532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van't Hoog A.H., Langendam M.W., Mitchell E. A systematic review of the sensitivity and specificity of symptom- and chest- radiography screening for active pulmonary tuberculosis in HIV-negative persons and persons with unknown HIV status. 2013. https://www.who.int/tb/Review2Accuracyofscreeningtests.pdf [DOI] [PMC free article] [PubMed]
- 13.Claassens M.M., Van Schalkwyk C., Floyd S. Symptom screening rules to identify active pulmonary tuberculosis: findings from the Zambian South African Tuberculosis and HIV/AIDS Reduction (ZAMSTAR) trial prevalence surveys. PLoS One. 2017;12:1–12. doi: 10.1371/journal.pone.0172881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.World Health Organization Guidelines for intensified tuberculosis case-finding and isoniazid preventive therapy for people living with HIV in resource- constrained settings. World Health. 2011;01:142. ISBN 978 92 4 150070 8. [Google Scholar]
- 15.Getahun H., Kittikraisak W., Heilig C.M. Development of a standardized screening rule for tuberculosis in people living with HIV in resource-constrained settings : individual participant data meta-analysis of observational studies. PLoS Med. 2011;8 doi: 10.1371/journal.pmed.1000391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Steingart K.K.R., Ng V., Henry M. Sputum processing methods to improve the sensitivity of smear microscopy for tuberculosis: a systematic review. Lancet Infect Dis. 2006;6:664–674. doi: 10.1016/S1473-3099(06)70602-8. [DOI] [PubMed] [Google Scholar]
- 17.World Health Organization . WHO; Geneva: 2013. Automated real-time nucleic acid amplification technology for rapid and simultaneous detection of tuberculosis and rifampicin resistance : Xpert MTB / RIF system for the diagnosis of pulmonary and extrapulmonary TB in adults and children: policy update.http://www.who.int/tb/publications/xpert-mtb-rif-assay-diagnosis-policy-update/en/ [PubMed] [Google Scholar]
- 18.Albert H., Nathavitharana R.R., Isaacs C. Development, roll-out and impact of Xpert MTB/RIF for tuberculosis: what lessons have we learnt and how can we do better? Eur Respir J. 2016;48:516–525. doi: 10.1183/13993003.00543-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.World Health Organization . WHO/HTM/TB/; Geneva: 2007. Improving the diagnosis and treatment of smear-negative and extrapulmonary TB: recommendations for HIV-prevalent and resource-constrained settings.https://apps.who.int/iris/handle/10665/69463 2007.379. [Google Scholar]
- 20.Medecins Sans Frontieres . 2014. Tuberculosis-pratical guide for clinicians, nurses, laboratory technicians and medical auxiliaries. [Google Scholar]
- 21.World Health Organization . WHO/CDS/CPC/TB/; Geneva: 1999. What is DOTS ? A guide to understanding the WHO-recommended TB control strategy known as DOTS; p. 99.270.https://apps.who.int/iris/handle/10665/65979 [Google Scholar]
- 22.Toman K.. World Health Organization; Geneva: 1979. Tuberculosis case-finding and chemotherapy: questions and answers. [Google Scholar]
- 23.World Health Organization . 2016. Chest radiography in tuberculosis detection: sSummary of current WHO recommendations and guidance on programmatic approaches.https://apps.who.int/iris/handle/10665/252424 Geneva: [Google Scholar]
- 24.Nguyen D.T.M., Bang N.D., Hung N.Q. Yield of chest radiograph in tuberculosis screening for HIV-infected persons at a district-level HIV clinic. Int J Tuberc Lung Dis. 2016;20:211–217. doi: 10.5588/ijtld.15.0705. [DOI] [PubMed] [Google Scholar]
- 25.Somashekar N., Chadha V.K., Praseeja P. Role of pre-Xpert screening using chest X-ray in early diagnosis of smear-negative pulmonary tuberculosis. Int J Tuberc Lung Dis. 2014;18:1243–1244. doi: 10.5588/ijtld.14.0141. [DOI] [PubMed] [Google Scholar]
- 26.Rahman T., Codlin A.J., Mahfuzur R. An evaluation of automated chest x-ray reading software for tuberculosis screening among public and private sector patients. Eur Respir J. 2017;49 doi: 10.1183/13993003.02159-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Onozaki I., Law I., Sismanidis C. National tuberculosis prevalence surveys in Asia, 1990–2012: an overview of results and lessons learned. Trop Med Int Heal. 2015;20:1128–1145. doi: 10.1111/tmi.12534. [DOI] [PubMed] [Google Scholar]
- 28.Pande T., Pai M., Khan F.A. Use of chest radiography in the 22 highest tuberculosis burden countries. Eur Respir J. 2015;46:1816–1819. doi: 10.1183/13993003.01064-2015. [DOI] [PubMed] [Google Scholar]
- 29.World Health Organization Tuberculosis Prevalence Surveys: a Handbook. Geneva: 2011 https://apps.who.int/iris/handle/10665/44481 [Google Scholar]
- 30.Kent P.T., Kubica G.P.. U.S. Department of Health and Human Services, Public Health Service, Centers for Disease Control; Atlanta, Ga: 1985. Public health Mycobacteriology: a guide for the level III laboratory. [Google Scholar]
- 31.Pheiffer C., Carroll N.M., Beyers N. Time to detection of Mycobacterium tuberculosis in BACTEC systems as a viable alternative to colony counting. Int J Tuberc Lung Dis. 2008;12:792–798. [PubMed] [Google Scholar]
- 32.Vandenbroucke J.P., Elm E.Von, Altman D.G. Strengthening the reporting of observational studies in epidemiology (STROBE): explanation and elaboration. PLoS Med. 2007;4:e297. doi: 10.1371/journal.pmed.0040297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van't Hoog A.H., Laserson K.F., Githui W.A. High prevalence of pulmonary tuberculosis and inadequate case finding in rural western Kenya. Am J Respir Crit Care Med. 2011;183:1245–1253. doi: 10.1164/rccm.201008-1269OC. [DOI] [PubMed] [Google Scholar]
- 34.Hoa N.B., Cobelens F.G.J., Sy D.N. Yield of interview screening and chest X-ray abnormalities in a tuberculosis prevalence survey. Int J Tuberc Lung Dis. 2012;16:762–767. doi: 10.5588/ijtld.11.0581. [DOI] [PubMed] [Google Scholar]
- 35.Churchyard G.J., Fielding K.L., Lewis J.J. Symptom and chest radiographic screening for infectious tuberculosis prior to starting isoniazid preventive therapy: yield and proportion missed at screening. AIDS. 2010;24:S19–S27. doi: 10.1097/01.aids.0000391018.72542.46. [DOI] [PubMed] [Google Scholar]
- 36.Den Boon S., White N.W., Van Lill S.W.P. An evaluation of symptom and chest radiographic screening in tuberculosis prevalence surveys. Int J Tuberc Lung Dis. 2006;10:876–882. 16898372. [PubMed] [Google Scholar]
- 37.Pande T., Pai M., Khan F.A. Use of chest radiography in the 22 highest tuberculosis burden countries. Eur Respir J. 2015;46:1816–1819. doi: 10.1183/13993003.01064-2015. [DOI] [PubMed] [Google Scholar]
- 38.Creswell J., Rai B., Wali R. Introducing new TB diagnostics - the impact of Xpert MTB RIF testing on case notifications in Nepal. Int J Tuberc Lung Dis. 2015;19:1–9. doi: 10.5588/ijtld.14.0775. [DOI] [PubMed] [Google Scholar]
- 39.Creswell J., Codlin A.J., Andre E. Results from early programmatic implementation of Xpert MTB/RIF testing in nine countries. BMC Infect Dis. 2014;14:2. doi: 10.1186/1471-2334-14-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lawn S.D., Kerkhoff A.D., Vogt M. Characteristics and early outcomes of patients with Xpert MTB/RIF-negative pulmonary tuberculosis diagnosed during screening before antiretroviral therapy. Clin Infect Dis. 2012;54:1071–1079. doi: 10.1093/cid/cir1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lawn S.D., Brooks S.V.., Kranzer K. Screening for HIV-associated tuberculosis and rifampicin resistance before antiretroviral therapy using the Xpert MTB/RIF assay : a prospective study. PLoS Med. 2011;8 doi: 10.1371/journal.pmed.1001067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sohn H., Aero A.D., Menzies D. Xpert MTB/RIF testing in a low tuberculosis incidence, high-resource setting: limitations in accuracy and clinical impact. Clin Infect Dis. 2014;58:970–976. doi: 10.1093/cid/ciu022. [DOI] [PubMed] [Google Scholar]
- 43.Henostroza G., Harris J.B., Chitambi R. High prevalence of tuberculosis in newly enrolled HIV patients in Zambia: need for enhanced screening approach. Int J Tuberc Lung Dis. 2016;20:1033–1039. doi: 10.5588/ijtld.15.0651. [DOI] [PubMed] [Google Scholar]
- 44.Dorman S.E., Schumacher S.G., Alland D. Xpert MTB/RIF Ultra for detection of Mycobacterium tuberculosis and rifampicin resistance: a prospective multicentre diagnostic accuracy study. Lancet Infect Dis. 2017;3099:1–9. doi: 10.1016/S1473-3099(17)30691-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mbu E.T., Sauter F., Zoufaly A. Tuberculosis in people newly diagnosed with HIV at a large HIV care and treatment center in Northwest Cameroon: burden, comparative screening and diagnostic yields, and patient outcomes. PLoS One. 2018;13:86–96. doi: 10.1371/journal.pone.0199634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dowdy D.W., Basu S., Andrews J.R. Is passive diagnosis enough? The impact of subclinical disease on diagnostic strategies for tuberculosis. Am J Respir Crit Care Med. 2013;187:543–551. doi: 10.1164/rccm.201207-1217OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Corbett E.L., MacPherson P. Tuberculosis screening in high human immunodeficiency virus prevalence settings: turning promise into reality. Int J Tuberc Lung Dis. 2013;17:1125–1138. doi: 10.5588/ijtld.13.0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Claassens M.M., Schalkwyk C.Van, Dunbar R. Patient diagnostic rate as indicator of tuberculosis case detection, South Africa. Emerg Infect Dis. 2016;22:535–537. doi: 10.3201/eid2203.151618. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.