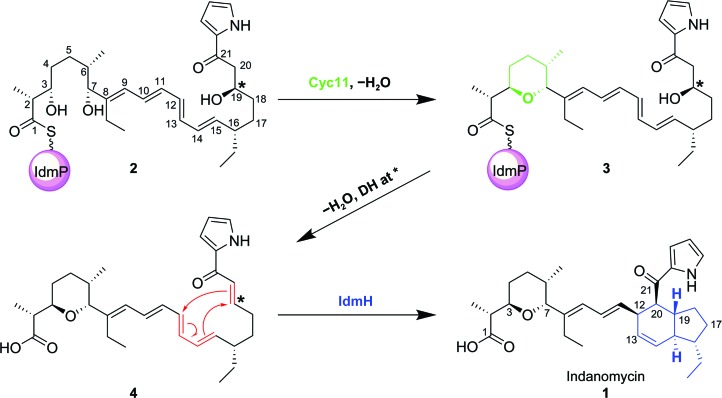
Figure 1.
The proposed mechanism for indanomycin maturation through the formation of tetrahydropyran (green) and indane (blue) rings. After ‘starter’ pyrrole biosynthesis, the polyketide is built through the actions of the five Idm ORFs IdmL–P, and the final polyketide chain is left attached to IdmP. Cyc11 is thought to mediate the formation of the tetrahydropyran ring through a direct nucleophilic replacement to generate 3 (Li et al., 2009 ▸). This reaction could then be followed by hydrolysis to release 4 from the IdmP subunit. Finally, indane-ring formation is thought to be mediated by a putative cyclase, IdmH (Li et al., 2009 ▸). For [4+2] cycloaddition to occur, C19 of 2 (denoted with an asterisk) needs to be dehydrated to produce a double bond to act as the dienophile (Rommel et al., 2011 ▸). Since the second PKS module does not contain a dehydratase domain, a hypothetical dehydration step was included between intermediates 3 and 4. Adapted from Li et al. (2009 ▸).