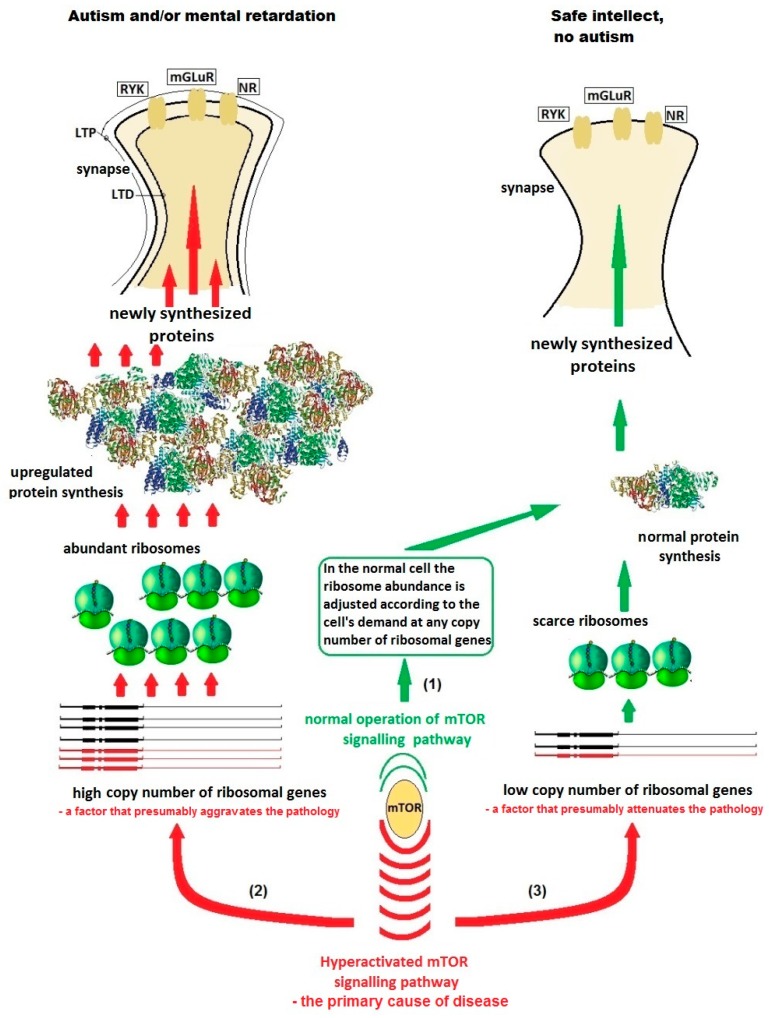
Figure 2.
A hypothetical scheme of the influence of ribosomal gene (RG) dosage in the genome of a non-carrier (green text and arrows) and a carrier (red text and arrows) of a monogenic autism mutation on the disease severity. The idea is that low RG copy numbers that lead to ribosome underabundance would attenuate the signs of monogenic autism caused by excessive protein production in the dendrites. (1) Without mutation, the copy number of ribosomal genes makes no difference. The cell produces such quantity of ribosomes and proteins as required for its functioning at the moment, via switching on and off the ribosomal DNA (rDNA) transcription according to demand. (2) A mutation resulting in hyper-activation of mTOR signaling pathway, provided that the cell has a sufficient quantity of ribosomal genes in the genome, leads to elevated rDNA transcription and hyper-activation of local (dendritic) translation resulting in overproduction of some proteins. The protein overproduction results in aberrant synapse morphology and defect LTD/LTP processes in the synaptic networks. Autism and (or) mental retardation develops. (3) I hypothesize here that formes frustes of the monogenic syndromes with safe intellect are only possible in case of the presence of a small number of copies of ribosomal genes in the genome. In that case, the ribosomal genes, despite hyperactivation, are unable to produce so much rRNA and, consequently, ribosomes, so that the local protein synthesis in dendrites would be measurably disturbed. Abbreviations: LTD/LTP—long-term depression/potentiation; RYK—receptor-like tyrosine kinase; mGLuR—metabotropic glutamate receptor; NR—N-methyl-d-aspartate receptor.