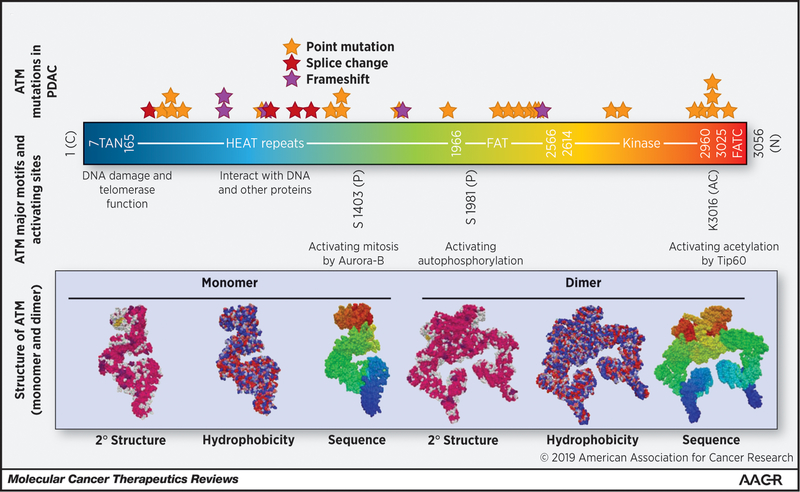
Figure 2: ATM Structure-function domains and frequent mutations in PDAC.
ATM has several important domains that are critical for ATM function as either a monomer, dimer or both. The TAN domain is critical for telomerase function and recruitment to DSBs. This recruitment is also dependent on interactions between the ATM HEAT repeats and NBS1 (part of the MRN complex)(31,34). The FAT domain normally inhibits the kinase activity as a dimer, but after DNA damage induced autophosphorylation at S1981 and subsequent dissociation of the dimer the kinase domain becomes active (36). The FATC domain is critical for interaction with TIP60, TIP60 acetylation of ATM at K3016 is necessary for ATM activation. Mutational analysis of PDAC patients with ATM mutations from Cbio-portal (date of accession 01/21/2019) did not show significant clustering or hotspot mutations in ATM, but the number of patients was low (N=34).