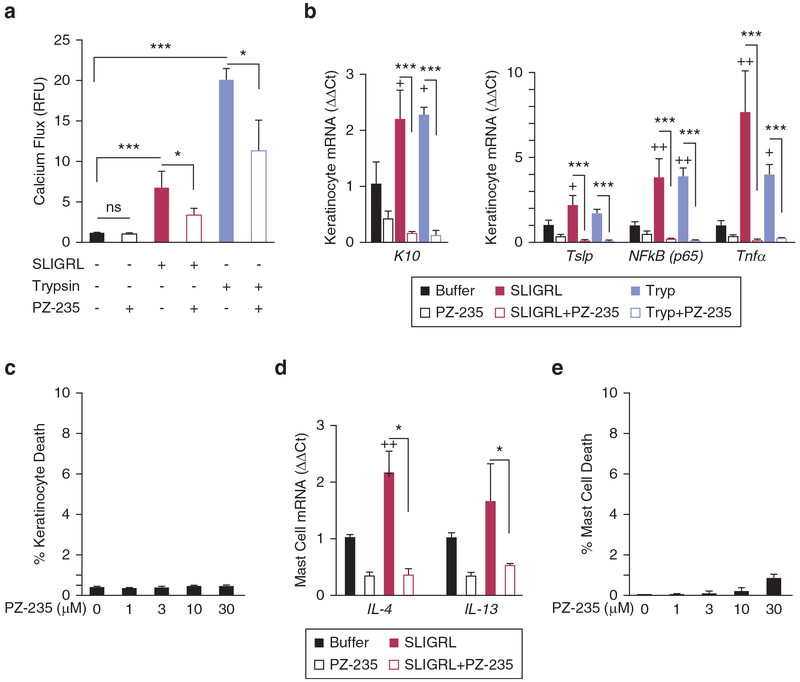
Figure 1. PZ-235 pepducin suppresses PAR2 calcium responses and expression of inflammatory mediators of atopic dermatitis in keratinocytes.
(a) The effect of PZ-235 on PAR2 signaling was measured in differentiated normal human epidermal keratinocytes (NHEKs) using a calcium (calcium-4 dye) flux assay. Keratinocytes were stimulated with PAR2 agonist peptide SLIGRL (3 μmol/L) or the PAR2-activating protease trypsin (10 nmol/L) in the presence of PZ-235 (3 μmol/L) or vehicle control (−). (b) PAR2 up-regulates keratinocyte differentiation marker and expression of inflammatory mediators in human keratinocytes which is completely suppressed by PZ-235. Treatment of differentiated NHEKs with PAR2 agonists SLIGRL (3 μmol/L) and trypsin (10 nmol/L) for 8 hours increases expression of the differentiation marker keratin-10 (K10) and atopic dermatitis inflammatory markers NF-κB (p65), Tnfα, and Tslp by quantitative PCR. Quantitative mRNA levels (ΔΔCt) were normalized to Gapdh. (c) NHEK keratinocytes were treated with 0–30 μmol PZ-235 as indicated for 8 hours at 37 °C, and dead cells were quantified by staining with propidium iodide for 15 minutes at room temperature before analysis of fluorescence by flow cytometry in duplicate for each condition. (d) Atopic dermatitis-related markers IL4 and IL13 mRNA were measured in HMC1 mast cells after 24-hour incubation with SLIGRL (3 μmol/L) in the presence or absence of 3 μmol/L PZ-235. (e) HMC1 mast cells were treated with 0–30 μmol/L PZ-235 as indicated for 24 hours at 37 °C, and dead cells were quantified by staining with propidium iodide for 15 minutes at room temperature before analysis of fluorescence by flow cytometry in duplicate for each condition. Means ± standard error of the mean, n = 3 are shown. *P < 0.05, ***P < 0.001 by t test; +P < 0.05, ++P < 0.01 versus individual buffer control by Dunnett test. M, mol/L; RFU, relative fluorescence unit; Tryp, trypsin.