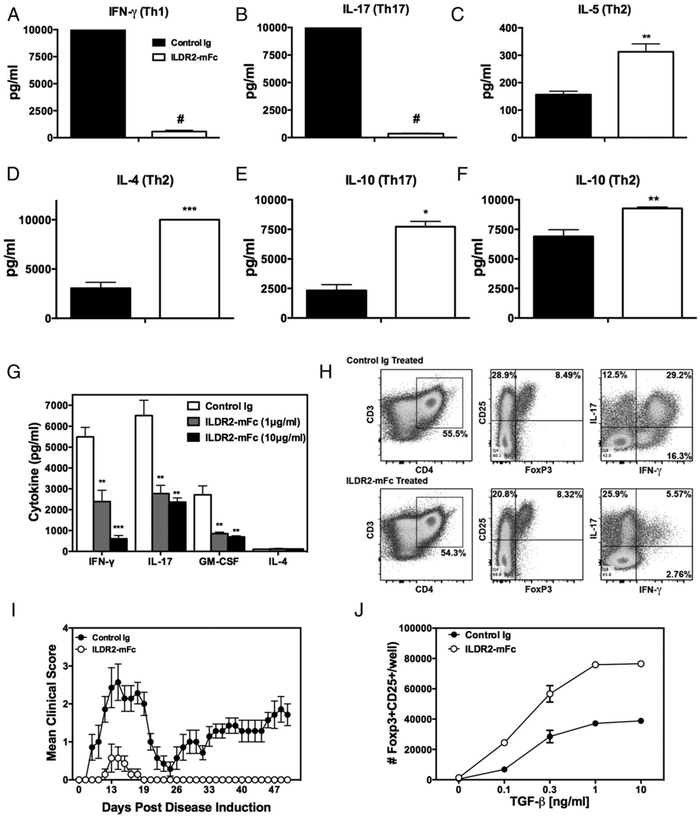
FIGURE 3.
ILDR2-mFc downregulates Th1 and Th17 differentiation and enhances Th2 and Treg differentiation in vitro. Naive CD4+ T cells isolated from DO11.10 mice were activated with OVA323–339 peptide (20 μg/ml) in the presence of irradiated APCs under Th1-, Th17-, or Th2-driving conditions, as described in Materials and Methods. Soluble ILDR2-mFc or control Ig (mIgG2a) (5 mg/ml) was added to these cultures. (A–F) Supernatants were collected after 72 h and analyzed for cytokine production via LiquiChip. Lymph node cells from PLP139–151/CFA primed mice were reactivated ex vivo with PLP139–151 in the presence of ILDR2-mFc or control Ig. The level of cytokine secretion (G) was assessed in triplicate, and the phenotype of the resultant cells was assessed via flow cytometry (H). These ex vivo-reactivated blasts were transferred to naive SJL/J mice and evaluated for transfer R-EAE induction (I). (J) Freshly isolated CD4+CD25− T cells were activated for 4 d with plate-bound anti-CD3 (2 μg/ml) and coimmobilized with 10 μg/ml ILDR2-mFc or control Ig (mIgG2a), in the presence of soluble anti-CD28 (1 μg/ml), with IL-2 (5 ng/ml) over the indicated range of TGF-β concentrations. The number of CD25+Foxp3+ cells was determined via FACS. Data represent mean ± SD of duplicate wells. One representative experiment of two or three independent experiments is presented for each experimental set. *p < 0.05, **p < 0.01, ***p < 0.001, #p < 0.0001 ILDR2-mFc versus control Ig.