Figure 1.
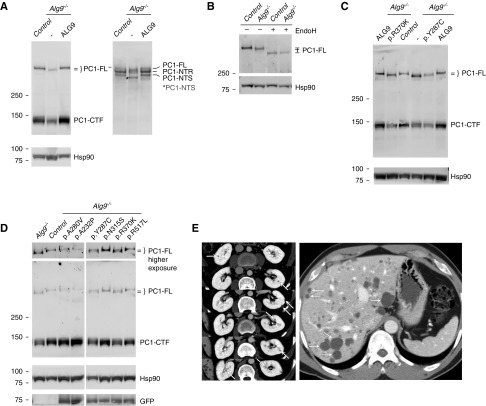
Alg9 loss causes abnormal biogenesis of PC1. (A) Immunoblots of cell lysate with anti-HA (left panel) and anti-LRR PC1–N-terminal antibody (7e12) (right panel) show quantitative decrease in PC1–C-terminal fragment (PC1-CTF) and the mature EndoH-resistant fraction of PC1–N-terminal fragment (PC1-NTR), as well as faster migration of PC1 full-length (PC1-FL) and the immature EndoH-sensitive PC1–N-terminal fragment (PC1-NTS) in Alg9−/− cells compared with controls. Re-expression of human ALG9-GFP in Alg9−/− cells rescues both the quantitative and migration differences (third lane). (B) Cell lysate was treated with EndoH and blotted with anti-HA. The migration difference of PC1-FL between control and Alg9−/− cell lysates is eliminated after EndoH treatment, showing that there is altered glycosylation of PC1 in the absence of Alg9. (C and D) Anti-HA immunoblots of cell lysate from Alg9−/− cells with or without stable re-expression of human ALG9-GFP with either wild-type (ALG9) or the indicated missense variants. The known pathogenic missense mutation, p.Y287C, and the experimental missense variant, p.R370K, do not rescue the PC1 phenotype in Alg9−/− cells. (D) The other experimental missense variants rescue the PC1 phenotypes. This bioassay identifies p.R370K as a deleterious missense mutation and p.A232P, p.A280V, pN315S, and p.R517L as benign variants. (E) Imaging for YU202 with ALG9-p.R370K. Serial CT scan sections show multiple small kidney cysts (arrows) many of which are likely hemorrhagic/proteinaceous (left panel) and innumerable liver cysts (right panel).