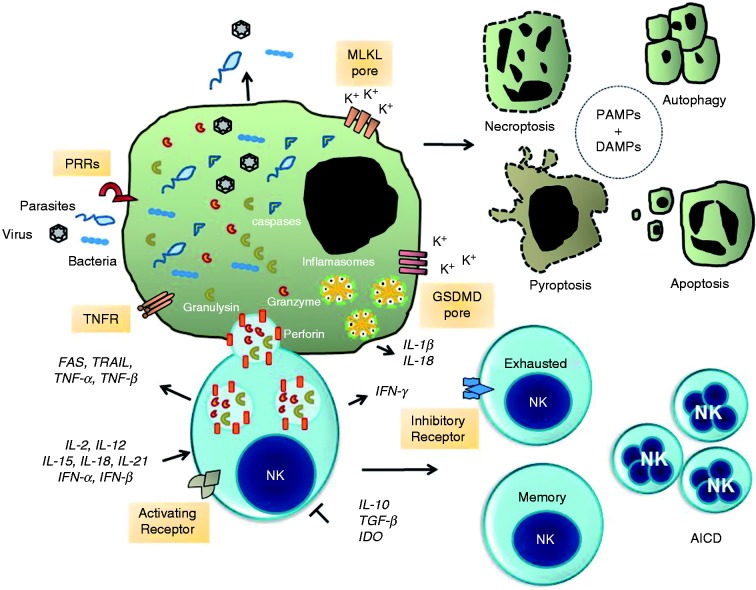
Figure 1.
NK cell-mediated signaling pathways to intracellular pathogen and infected cell death. The formation of immunological synapse between a NK cell and the target cell occurs upon identification of specific self-deficiencies by an array of either paired activating (AR) or inhibitory membrane receptors (IR) and their ligands. NK cells release cytotoxic granules containing the pore-forming protein perforin, granulysin and serine proteases known as granzymes. Granzymes promote the cleavage and activation of a family of protease known as caspases. Caspases promote proteolytic cleavage of cellular substrates leading to apoptosis. Alternatively, the assembly of the channels and pores formed by MLKL and GSDMD promote the exchange of Ca2+, Na+, and K+ ions that contribute to osmotic perturbation and ultimately cell death by necroptosis or pyroptosis, respectively. Infected cells may die by autophagy, which is a process that can inhibit or activate programmed cell death. Finally, sustained expression and activation of IRs promotes NK cell exhaustion whereas over-activation of ARs leads to activation-induced cell death (AICD). Concomitantly, survival NK cell clones expand to make a memory repertoire of NK cells for a specific pathogen. Extracellular and intracellular pathogens or their products (PAMPs and DAMPs) activate the inflammasome signaling pathways and production of pro-inflammatory cytokine IL-1β and IL-18. Production of the immune suppressive cytokines TGF-β and IL-10 and indoleamine 2,3-dioxygenase (IDO) abolish NK cell activation and tissue damage. Abbreviations: GM-CSF, granulocyte-macrophage CSF; CD95/FasL (Fas ligand); TRAIL, TNF-related apoptosis-inducing ligand; TNFR, TNF receptor; DAMPs, danger-associated molecular patterns; MLKL, mixed lineage kinase domain-like; GSDMD, gasdermin D.