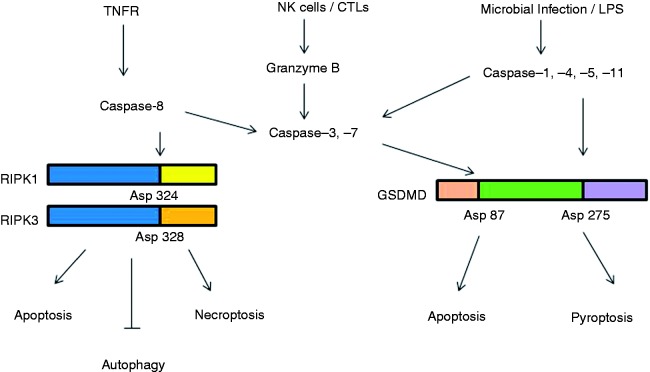
Figure 2.
Caspases-mediated cleavage and the switch in programmed cell death signaling pathways. In response to pathogen invasion, RIPK1 is activated either directly, by ligation of the TNF receptor, or indirectly, by ligation of TLRs (via TRIF) or IFN receptors. Cleavage of RIPK1 at Asp324 and RIPK3 at Asp328 by caspase-8 induces apoptosis. Inhibition of caspase-8 by chemical or viral inhibitors leads to the formation of RIPK-1/RIPK-3 complex (necrosome). RIP3 phosphorylates its substrate MLKL and this event drives MLKL oligomerization and translocation to the plasma membrane, leading to necroptosis. Silencing of RIPK1 under both normal and starvation conditions enhances autophagy. NK cells and cytotoxic lymphocytes (CTLs) deliver cytotoxic molecules, including perforin, granulysin and granzymes (Gzm), into the cytosol of infected cells. Gzm B mediates the cleavage and activation of caspase-3 and -7. Microbial pathogens and inflammatory agents or LPS via interaction cytosolic sensor proteins or with caspase -4, -5 and -11, can activate the canonical and non-canonical inflammasome pathways, respectively. Inflammasome complexes activate caspase-1, which promotes the cleavage and activation of IL-1β and IL-18. In parallel, caspase-1 cleaves the protein gasdermin-D (GSDMD) at Asp275 to induce pyroptosis cell death events. If GSDMD is cleaved at Asp87 by caspase-3 or -7 pyroptosis events are put on hold and cells switch to apoptosis.