Background:
Tenofovir disoproxil fumarate coformulated with emtricitabine (TDF/FTC) was shown to be effective in preventing HIV acquisition when used for pre-exposure prophylaxis (PrEP), but questions have arisen regarding optimal PrEP implementation strategies.
Methods:
A narrative review of literature since 2010 regarding PrEP effectiveness, implementation, and new prevention modalities was undertaken to summarize lessons learned, and to review potential benefits and challenges.
Results:
Although daily TDF/FTC is safe, well tolerated, and highly effective in preventing HIV transmission, it has been initiated by only 200,000 Americans, and a comparable number of individuals in other countries, meaning that 80%–90% of those at greatest risk globally have not benefitted yet. Barriers to PrEP uptake have included medication and care costs, anticipated side effects, stigma, and unsupportive health care systems. Innovations to increase PrEP uptake and adherence have included engaging nonmedical staff (eg, pharmacists, social workers, and peer navigators), economic assistance programs, and new technologies (eg, text messaging support and dedicated apps). Pericoital PrEP dosing seems to be effective in preventing HIV transmission among men who have sex with men, but has not been evaluated in women. Investigational PrEP approaches include antiretrovirals delivered by injection, implant, vaginal rings, rectal douches, and immunoprophylaxis. Some of these approaches may allow for infrequent dosing, whereas others may be more congruent with patterns of sexual behavior.
Conclusions:
PrEP has been shown to be safe and effective when used consistently, but new approaches to enhance uptake, adherence, and convenience with less-frequent dosing are under study, suggesting that new models and modalities will evolve to optimize impact.
Key Words: pre-exposure prophylaxis, PrEP, HIV prevention, antiretrovirals, high risk individuals, medication adherence
Since the demonstration that modern era highly active antiretroviral therapy (HAART) resulted in improved health outcomes for people living with HIV, we have learned that HAART renders individuals untransmissible if their viral load has been consistently undetectable for several months.1 Despite multiple efficacy trials demonstrating that the use of tenofovir disoproxil fumarate (TDF) with emtricitabine (FTC) for pre-exposure prophylaxis (PrEP) can decrease HIV incidence in high-risk populations,2–4 many wondered whether scaling up this intervention was necessary. However, although there has been a diminution in the rate of new HIV infections over the past few years5,6 due to improved access to care and improved therapies, the global community remains far away from achieving the goal of diagnosing 90% of new HIV infections, providing HAART for 90% of those infections, and achieving viral suppression in 90% of those treated by the year 2020.5 In fact, in 2017 alone, there were close to 2 million new HIV infections.5 Of the nearly 37 million people living with HIV in 2017, only 21.7 million people were receiving treatment, and less than 18 million were consistently virologically suppressed.5 Moreover, several modeling groups have found that a combination of scaling up antiretroviral therapy, achieving virologic suppression, and the addition of PrEP achieves the most rapid decreases in HIV incidence.7–10
Initial concerns about the use of PrEP for HIV prevention focused on questions about behavioral disinhibition and suboptimal adherence when available in the “real world.” Efficacy estimates in clinical trials ranged from 86% in the PROUD11 and IPERGAY12 studies to no demonstrated protection seen in FEM-PrEP13 and VOICE.14 However, post hoc analyses looking at drug levels found that adherence was linearly related to the level of protection, and that the better results of several of the men who have sex with men (MSM) studies were clearly related to higher levels of adherence. Since the approval of TDF/FTC for PrEP by the FDA and other regulatory bodies, the efficacy of PrEP seems even better in real-world settings.10,15,16 One explanation for those findings may be that participants who initiate PrEP in the “real world” were motivated to do so, whereas particularly in the initial clinical trials, the efficacy of PrEP had not been demonstrated, and individuals knew they had a chance of receiving a placebo; so, motivations for adherence may have been limited.
Despite these salutary findings, PrEP has not been approved in many countries, and scale up is slower than ideal. The pace has picked up in recent years in the United States, with more than 10000 PrEP initiations recently occurring on a monthly basis,17 but this means that in the United States, about 250,000 individuals have initiated PrEP, whereas the US Centers for Disease Control and Prevention estimates that over 1.1 million individuals are eligible18 (Fig. 1). Globally, there are now approximately 200,000 additional individuals who have initiated PrEP, with a diverse set of national leaders, including Brazil, Kenya, South Africa, Thailand, France, and the Netherlands.17
FIGURE 1.
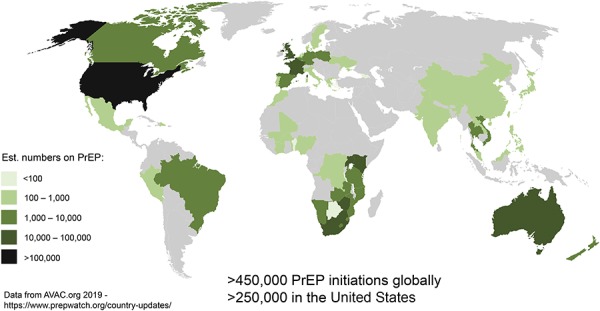
Global PrEP rollout.
Although the increasing rate of PrEP initiation has been encouraging, PrEP persistence remains a challenge, with more than half of the individuals not continuing on PrEP for more than a year (Gilead Sciences, written communication). The research to understand the factors associated with PrEP discontinuation needs further refinement because there are numerous factors to consider: structural issues range from costs of the medication or laboratory-monitoring tests, to judgmental attitudes on the part of health care workers, to informational barriers such as the fact that those who could benefit most from PrEP may unaware that it exists. Comorbid psychologic illness may also contribute to PrEP discontinuation.19 Thus, increasing PrEP scale up will need to address a wide range of challenges.
In the United States, there continues to be high levels of PrEP access disparities, exemplified by the observation that up to 44% of the people who would benefit from PrEP are African American, whereas only about 1% of those prescribed PrEP to date are African American.18 Although the HIV epidemic continues to grow most rapidly in the southern United States,20 many of these states have not expanded the Affordable Care act, and thus have not made PrEP more widely available.
There are attempts to address those social and structural issues by developing culturally tailored programs. HPTN 073 tested a theory-based program that included peer health care workers to assist in PrEP provision through a client-centered program of care coordination.21 Of the 226 participants in 3 US cities, the majority indicated a willingness to initiate PrEP when approached, and at the end of the year, most were retained in PrEP care. However, the overall HIV incidence in that population was 2.9%, which was higher than that in other similar studies, suggesting that additional refinements are needed. Another population that has demonstrated challenges with PrEP adherence are younger individuals,15 and despite a study evaluating 2 evidence-based behavioral interventions to support PrEP adherence, HIV incidence was approximately 6% in young MSM aged 15–17 years, and over 3% among those aged 18–22 years.22
In addition to the need to develop culturally tailored interventions for youth and people of color, as well as other key populations around the world, PrEP must be construed to be part of a broader sexual health package.23 Individuals who access PrEP frequently had not been using condoms in the first place,24 although there may be further behavioral disinhibition after PrEP initiation.25 The incidence of sexually transmitted infections (STI) after PrEP has either remained the same or increased.26,27 Thus, for PrEP to have optimal impact as a public health intervention, frequent screening and treatment for STI, as well as partner notification services, are essential parts of the “PrEP package.”28 When viewed as part of a larger biobehavioral intervention, PrEP can provide an opportunity to be a “gateway to care” similar to how accessing reproductive health counseling has led many otherwise healthy women into seeking other primary care services. A recent study at Fenway Health found that individuals who screened for STI and also initiated PrEP were more likely to receive a flu vaccination as well as tobacco and depression screenings.29
There are a variety of approaches now trying to address ways to implement PrEP optimally in diverse settings. Several US states and the District of Colombia have recognized that some of the challenges to PrEP uptake are due to having inadequate health insurance and/or high copays, so that these jurisdictions have developed drug assistance programs to facilitate PrEP access.30 Because busy clinicians in urban areas may not be optimally familiar with PrEP, several jurisdictions such as the New York City Department of Health have created academic detailing teams that go into busy clinics and provide education designed to increase PrEP scale up among generalists. Others are working on the development of algorithms to assist clinics through dropdown menus in electronic health records and other heuristics, to help providers identify appropriate PrEP candidates. It has also been pointed out that PrEP delivery is not complicated, and thus training other health professionals, such as pharmacists, may be a way to enhance PrEP scale up as well.31,32 Less expensive than training other health care professionals would be the development of cadres of health system navigators who can assist PrEP candidates in filling out the paperwork to participate in the subsidized drug assistance programs available to them, or other state-specific programs or forms of care cost subsidy. In addition, under evaluation are home-monitoring platforms, obviating the need for frequent clinic visits if an individual is stably maintained on PrEP.33
Studies evaluating new technologies are underway because many of the people at risk for HIV have cell phones and often use apps and other internet-based tools. Text message interventions have been used to augment PrEP adherence.34–36 Apps are being developed to enhance adherence, give feedback on sexual behavior and drug levels, and provide information on where HIV testing sites and PrEP facilities are located.37,38 Other modalities for PrEP monitoring are under study, including the use of hair samples, dried blood spots, and ingested electronic pill sensors.39 The use of such tools will be essential to further improvements in PrEP adherence. In addition, such tools may help address what has been called the “purview paradox” in which generalists think that PrEP is an issue for HIV specialists to address, and HIV specialists feel they are more comfortable only taking care of people living with HIV and not having to provide primary care.40
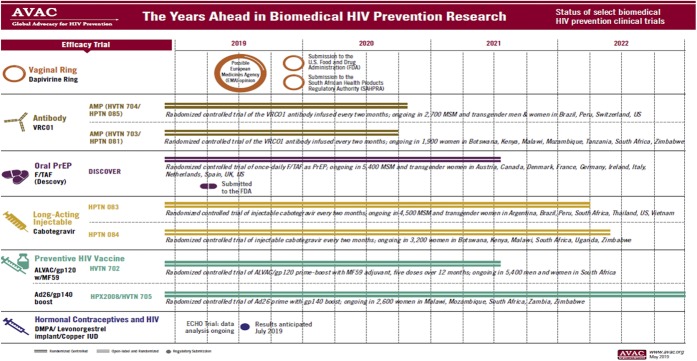
The need for an integrated and robust biobehavioral research agenda is very clear because PrEP will be moving from “1.0” to “2.0” and beyond over the next few years (Fig. 2). Daily PrEP dosing has been shown to be efficacious in all populations where it has been studied, but “on-demand” PrEP has been shown to work well for MSM as long as they take 2 doses within 24 hours of sex and a pill a day for 2 days afterward.41 Such an event-driven PrEP has enabled a large cohort of French and Canadian MSM to use almost half the amount of medication than that of a daily regimen without any new seroconversions.42 The pharmacology of this approach for cis- and trans-gender women is not fully understood, and thus it is not recommended that they consider this on-demand dosing scheme.
FIGURE 2.
The years ahead in biomedical HIV prevention research.
Additional important considerations include side-effect profiles. Initial concerns of renal toxicity and bone disease related to TDF/FTC PrEP have not been borne out in clinical trials, which have demonstrated infrequent renal and bone abnormalities. A newer form of tenofovir (tenofovir alafenamide) has been demonstrated to reduce HIV incidence at least as effectively as a TDF-based regimen43 and has had more favorable findings related to bone and renal safety.44 Other approaches currently in trial include vaginal rings, implants, and topical microbicides45 (Fig. 1). Injectable PrEP, which may be able to be given as infrequently as every 8 weeks, is under study in HVTN 083 and 084, which are evaluating the integrase strand transfer inhibitor cabotegravir versus daily TDF/FTC. In addition to chemoprophylaxis with antiretrovirals, 2 large efficacy trials of a broadly acting neutralizing antibody (VRC01) evaluating immunoprophylaxis are underway in the United States, Latin America, and Africa.
Thus, PrEP is continuing to roll out despite many structural challenges. There is an important research agenda for behavioral and social scientists as PrEP and treatment as prevention become increasingly recognized as core elements of the international global AIDS strategy. The reason for the primacy of social and behavioral science in this setting is that the pills do not take themselves, and understanding how best to optimize access, risk perception, and adherence require sophisticated and multidisciplinary teams. For treatment as prevention and PrEP to be successful, research and public health programs will have to recognize concomitant social and structural issues, particularly related to poverty and housing instability, individuals' responses to being part of stigmatized populations (eg, depression and substance use), and potential biological cofactors such as STI. The advent of pills that are extremely effective, safe, and well tolerated for treatment and prevention has represented a major advance in addressing the global HIV pandemic, but these tools will be for naught if culturally appropriate programs that address the community and individual reasons for risk, nonengagement, and nonadherence are not rapidly developed, studied, integrated, and implemented.
Footnotes
This publication resulted in part from research supported by the Penn Center for AIDS Research (CFAR) (P30 AI 045008 - Ronald Collman, PI), the Penn Mental Health AIDS Research Center (PMHARC) (P30 MH 097488 - Dwight Evans, PI) and the CFAR Social & Behavioral Science Research Network National Scientific Meeting (SBSRN) (R13 HD 074468 - Michael Blank, PI).
K.H.M. has received unrestricted research grants to study antiretrovirals for prevention from Gilead Sciences and ViiV Healthcare. This work is partially supported by the Bio-behavioral and Community Science Core of the Harvard Center for AIDS Research (P30AI060354). The remaining author has no conflicts of interest to disclose.
REFERENCES
- 1.Quinn TC, Wawer MJ, Sewankambo N, et al. Viral load and heterosexual transmission of human immunodeficiency virus type 1: Rakai Project Study Group. N Engl J Med. 2000;342:921–929. [DOI] [PubMed] [Google Scholar]
- 2.Baeten JM, Donnell D, Ndase P, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012;367:399–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thigpen MC, Kebaabetswe PM, Paxton LA, et al. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med. 2012;367:423–434. [DOI] [PubMed] [Google Scholar]
- 4.Grant RM, Lama JR, Anderson PL, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363:2587–2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.UNAIDS: Global HIV & AIDS Statistics—2018 Fact Sheet. Available at: http://www.unaids.org/en/resources/fact-sheet. Accessed July 1, 2019. [Google Scholar]
- 6.Nwokolo N, Hill A, McOwan A, et al. Rapidly declining HIV infection in MSM in central London. Lancet HIV. 2017;4:e482–83. [DOI] [PubMed] [Google Scholar]
- 7.Blaizot S, Huerga H, Riche B, et al. Combined interventions to reduce HIV incidence in KwaZulu-Natal: a modelling study. BMC Infect Dis. 2017;17:522–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Akudibillah G, Pandey A, Medlock J. Maximizing the benefits of ART and PrEP in resource-limited settings. Epidemiol Infect. 2017;145:942–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cremin I, Alsallag R, Dybul M, et al. The new role of antiretrovirals in combination HIV prevention: a mathematical modelling analysis. AIDS. 2013;27:447–458. [DOI] [PubMed] [Google Scholar]
- 10.Smith JA, Anderson SJ, Harris KL, et al. Maximising HIV prevention by balancing the opportunities of today with the promises of tomorrow: a modelling study. Lancet HIV. 2016;3:e289–e296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCormack S, David DT, Desai M, et al. Pre-exposure prophylaxis to prevent the acquisition of HIV-1 infection (PROUD): effectiveness results from the pilot phase of a pragmatic open-label randomised trial. Lancet. 2016; 387: 53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Molina JM, Capitant C, Spire B, et al. On-demand preexposure prophylaxis in men at high risk for HIV-1 infection. N Engl J Med. 2015;373:2237–2246. [DOI] [PubMed] [Google Scholar]
- 13.Van Damme L, Corneli A, Ahmed K, et al. Preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2012;367:411–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marrazzo JM, Ramjee G, Richardson BA, et al. Tenofovir-based preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2015;372:509–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grulich A, Guy RJ, Amin J, et al. Rapid reduction in HIV diagnoses after targeted PrEP implementation in NSW, Australia (Abstract 88). Presented at: 25th Conference on Retroviruses and Opportunistic Infections; March 4–7, 2018; Boston, MA.
- 16.Buchbinder SP, Cohen SE, Hecht J, et al. Getting to zero new HIV diagnoses in San Francisco: what will it take? (Abstract 87). Presented at: 25th Conference on Retroviruses and Opportunistic Infections; March 4–7, 2018; Boston, MA.
- 17.PrEPWatch: Country Updates: Available at: https://www.prepwatch.org/in-practice/country-updates/. Accessed July 1, 2019. [Google Scholar]
- 18.Centers for Disease Control and Prevention. Press Release: HIV Prevention Pill Not Reaching Most Americans Who Could Benefit—Especially People of Color. 2018. Available at: https://www.cdc.gov/nchhstp/newsroom/2018/croi-2018-PrEP-press-release.html. [Google Scholar]
- 19.Velloza J, Baeten JM, Haberer J, et al. Effect of depression on adherence to oral PrEP among men and women in East Africa. JAIDS. 2018;79:330–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Elopre L, Kudroff K, Westfall AO, et al. The right people, right places, and right practices: disparities in PrEP access among African American men, women, and MSM in the deep South. J Acquir Immune Defic Syndr. 2017;74:56–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wheeler DP, Fields SD, Beauchamp G, et al. Pre-exposure prophylaxis initiation and adherence among Black men who have sex with men (MSM) in three US cities: results from the HPTN 073 study. J Int AIDS Soc. 2019;22:e25223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hosek S, Celum C, Wilson CM, et al. Preventing HIV among adolescents with oral PrEP: observations and challenges in the United States and South Africa. J Int AIDS Soc. 2016;19:21107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mayer KH, Chan PA, Patel R, et al. Evolving models and ongoing challenges for HIV preexposure prophylaxis implementation in the United States. J Acquir Immune Defic Syndr. 2018;77:119–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gamarel KE, Golub SA. Intimacy motivations and pre-exposure prophylaxis (PrEP) adoption intentions among HIV-negative men who have sex with men (MSM) in romantic relationships. Ann Behav Med. 2015;49:177–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoenigl M, Morgan E, Franklin D, et al. Self-initiated continuation of and adherence to HIV pre-exposure prophylaxis (PrEP) after PrEP demonstration project roll-off in men who have sex with men: associations with risky decision making, impulsivity/disinhibition, and sensation seeking. J Neurovirol. 2019;25:324–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mayer KH, Maloney KM, Levine K, et al. Sociodemographic and clinical factors associated with increasing bacterial sexually transmitted infection diagnoses in men who have sex with men accessing care at a Boston Community Health Center (2005–2015). Open Forum Infect Dis. 2017;4:ofx214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scott HM, Klausner JD. Sexually transmitted infections and pre-exposure prophylaxis: challenges and opportunities among men who have sex with men in the US. AIDS Res Ther. 2016;13:5–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kojima N, Klausner JD. Improving management of sexually transmitted infections in those who use pre-exposure prophylaxis for human immunodeficiency virus infection. AIDS. 2018;32:272–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marcus JL, Levine K, Grasso C, et al. HIV preexposure prophylaxis as a gateway to primary care. Am J Public Health. 2018;108:1418–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Washington State Department of Health: Pre-exposure Prophylaxis Drug Assistance Program (PrEP DAP). Available at: https://www.doh.wa.gov/YouandYourFamily/IllnessandDisease/HIV/Prevention/PrEPDAP. Accessed July 1, 2019. [Google Scholar]
- 31.Patel R, Federman S, Tung E, et al. Pharmacy-based pre-exposure prophylaxis for the prevention of HIV: innovative service delivery models. Webinar presentation at: Centers for Disease Control and Prevention; June 22, 2017; Atlanta, GA.
- 32.Tung E, Thomas A, Allyson E, et al. Feasibility of a pharmacist-run HIV PrEP clinic in a community pharmacy setting. Conference on Retroviruses and Opportunistic Infections; February 13–16, 2017; Seattle, WA.
- 33.Siegler AJ, Mayer KH, Liu AY, et al. Developing and Assessing the feasibility of a home-based preexposure prophylaxis monitoring and support program. Clin Infect Dis. 2019;68:501–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lester RT, Ritvo P, Mills EJ, et al. Effects of a mobile phone short message service on antiretroviral treatment adherence in Kenya (WelTel Kenya1): a randomised trial. Lancet. 2010;376:1838–1845. [DOI] [PubMed] [Google Scholar]
- 35.Mayer KH, Safren SA, Elsesser SA, et al. Optimizing pre-exposure antiretroviral prophylaxis Adherence in men who have sex with men: results of a pilot randomized controlled trial of “Life-Steps for PrEP”. AIDS Behav. 2017;21:1350–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fuchs JD, Stojanovski K, Vittinghoff E, et al. A mobile health strategy to support adherence to antiretroviral preexposure prophylaxis. AIDS Patient Care STDS. 2018;32:104–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Buchbinder SP, Liu AY. CROI 2015: Advances in HIV testing and prevention strategies. Top Antivir Med. 2015;23:8–27. [PMC free article] [PubMed] [Google Scholar]
- 38.Biello KB, Marrow E, Mimiaga MJ, et al. A mobile-based app (MyChoices) to increase uptake of HIV testing and pre-exposure prophylaxis by young men who have sex with men: protocol for a pilot randomized controlled trial. JMIR Res Protoc. 2019;8:e10694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chai PR, Castillo-Manchilla J, Buffkin E, et al. Utilizing an ingestible biosensor to assess real-time medication adherence. J Med Toxicol. 2015;11:439–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Krakower D, Ware N, Mitty JA, et al. HIV providers' perceived barriers and facilitators to implementing pre-exposure prophylaxis in care settings: a qualitative study. AIDS Behav. 2014;18:1712–1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Anderson PL, García-Lerma JG, Heneine W. Nondaily preexposure prophylaxis for HIV prevention. Curr Opin HIV AIDS. 2016;11:94–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Molina JM, Ghosn J, Béniguel L, et al. Incidence of HIV-infection in the ANRS prevenir study in paris region with daily or on-demand PrEP with TDF/FTC (WEAE0406LB). Presented at the AIDS 2018 Conference; July 25, 2019; Amsterdam, the Netherlands.
- 43.Hare CB, Coll J, Ruane P, et al. The phase 3 discover study: daily F/TAF or F/TDF for HIV pre-exposure prophylaxis (Abstract LB 0104). Presented at: Conference on Retroviruses and Opportunistic Infections; March 7, 2019; Seattle, WA.
- 44.Venter WDF, Fabian J, Feldman C. An overview of tenofovir and renal disease for the HIV-treating clinician. South Afr J HIV Med. 2018;19:817–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.HIV Prevention Trials. Available at: www.avac.org. Accessed May 5, 2019. [Google Scholar]