Abstract
Objective:
Secreted modular calcium-binding proteins (SMOCs) are extracellular glycoproteins of the secreted protein, acidic, and rich in cysteine-related modular calcium-binding protein family and include two isoforms, SMOC1 and SMOC2, in humans. Functionally, SMOCs bind to calcium for various cell functions. In this review, we provided a summary of the most recent advancements in and findings of SMOC1 and SMOC2 in development, homeostasis, and disease states.
Data sources:
All publications in the PubMed database were searched and retrieved (up to July 24, 2019) using various combinations of keywords searching, including SMOC1, SMOC2, and diseases.
Study selection:
All original studies and review articles of SMOCs in human diseases and embryo development written in English were retrieved and included.
Results:
SMOC1 and SMOC2 regulate embryonic development, cell homeostasis, and disease pathophysiology. They play an important role in the regulation of cell cycle progression, cell attachment to the extracellular matrix, tissue fibrosis, calcification, angiogenesis, birth defects, and cancer development.
Conclusions:
SMOC1 and SMOC2 are critical regulators of many cell biological processes and potential therapeutic targets for the control of human cancers and birth defects.
Keywords: Angiogenesis; Cancer; Cell cycle; Embryonic development; SMOCs; Secreted protein, Acidic and rich in cysteine
Introduction
The secreted protein, acidic and rich in cysteine (SPARC), also known as osteonectin (ON), or basement membrane-40, is an acidic extracellular matrix glycoprotein and plays an important role in bone mineralization, cell-matrix interactions, collagen binding, and bone remodeling.[1–3] Secreted modular calcium-binding proteins (SMOCs) are extracellular glycoproteins of the SPARC-related modular calcium-binding protein family with two isoforms, SMOC1 and SMOC2, in humans. SMOCs influence cell growth factor signaling, cell migration and proliferation, and angiogenesis.[4,5]SMOC1 was discovered in 2002, while SMOC2 was identified thereafter.[4,5] To date, their gene structures, patterns of expression, and functions have been precisely identified and studied for their involvement in multiple biological processes including embryonic development,[6] cell cycle progression,[7] cell attachment,[8] tissue fibrosis,[9,10] calcification,[11] angiogenesis,[12] and tumor development.[13,14] This review summarizes the recent advancements SMOC1 and SMOC2 and their role in embryonic development and homeostasis as well as human diseases.
SMOC Molecular Structures and Expression in Cells and Tissues
The full-length complementary DNA (cDNA) of SMOC1 was cloned in 2002 as a secreted modular glycoprotein and expressed in the basement membrane and the extracellular matrix of many tissues.[13] The SMOC1 gene is localized at chromosome 14q24.2 and encodes a protein of 434 amino acids with a molecular weight of 48,000.[4] SMOC1 protein contains five structural domains, including a follistatin-like domain, two thyroglobulin-like domains, a unique domain, and an helix-loop-helix motif (EF-hand) calcium-binding domain (or the extracellular calcium-binding [EC] domain). A subsequent study led by the same group of researchers also identified the SMOC2 gene by search expressed sequence tag sequence databases.[5] The SMOC2 gene is localized at chromosome 6q27 and shares 55% of DNA sequence homology with SMOC1.[5] SMOC2 has a predicted protein structure that contains similar domains to SMOC1, except for its own unique SMOC domain.[5] SMOC1 protein is highly expressed in the ovary, brain, thymus, heart, skeletal muscle, liver, and lung, while SMOC2 protein is expressed throughout the human body[4,5] and on basement membranes and the extracellular matrix.[7,15] Immunohistochemical analysis showed that SMOC1 was expressed in myocytes and as a component of the basement membrane in blood capillaries besides the cell surface.[4] Furthermore, SMOC1 protein can bind to collagen IV through its EC domain in the basement membrane.[4] The SMOC1 interaction with collagen IV indicates that SMOC1 distributes widely in the basement membrane; however, there are few studies to date demonstrating that SMOC1 interacts with SPARC in the same manner. In contrast, SMOC2 protein participates in the assembly of fibrils and is co-localized with fibronectin, the main component of the extracellular matrix.[5] Thus, SMOC2 acted as a component of fibrils and the characteristic of SMOC2 co-localization with fibronectin may not be typical for the extracellular proteins. SMOC2 has also been identified as a marker of intestinal stem cells,[16] implying that it may have multiple pleiotropic roles.[5,16] Taken together, although SMOC1 and SMOC2 shared high DNA sequence similarity, they have distinct functions in the human body.
Although recombinant SMOC proteins can be generated in Escherichia coli,[16] such recombinant SMOC proteins show little biological activity due to the lack of the glycosylation process in vitro. Thus, there is an urgent need to reconstitute functionally glycosylated SMOC proteins for future research.
SMOC Functions in Cells and Embryo Development
Embryonic development
A previous study analyzed the expression and regulation of SMOC1 and SMOC2 in fetal gonad/mesonephros complexes and revealed their possible role in the gonad and mesonephros development, that is, SMOC1 expression was elevated at approximately E10.75 in pre-sertoli and pre-granulosa cells but was significantly reduced in the Wilms tumor protein 1, splicing factor 1, and friend of GATA2 mutants. At embryonic day 13.5 (E13.5), SMOC1 expression was reduced in granulosa cells but persisted in Sertoli cells, indicating a sexually dimorphic requirement in cell lineage differentiation. In contrast, SMOC2 protein was expressed in Leydig cells and mesonephroi and Wnt4 mutant ovaries, while SMOC2 expression required functional Hedgehog signaling in the testes, mesonephroi, and kidneys.[17] SMOC1 was also reported to be essential for post-gastrulation development in Xenopus by inhibition of bone morphogenetic protein (BMP) signaling[18] and for ocular and limb development in both humans and mice.[19] Furthermore, during mouse embryogenesis, SMOC1 is expressed in the early mouse embryo at E7 in the entire endodermal basement membrane zone of the embryo proper.[20]SMOC1 messenger RNA (mRNA) is expressed in mesenchymal as well as epithelial cells deriving from all three germ layers in the early stages of mouse development. This broad and organ-specific SMOC1 expression underscores SMOC1's multi-functional roles in mouse embryogenesis.[20] Thomas et al[18] demonstrated in mice that SMOC2 mRNA was present in mid-gestation embryos, particularly in the forelimb, hindlimb, somites, and branchial arches.[7,21]SMOC2 mutations were associated with developmental dental defects.[22]
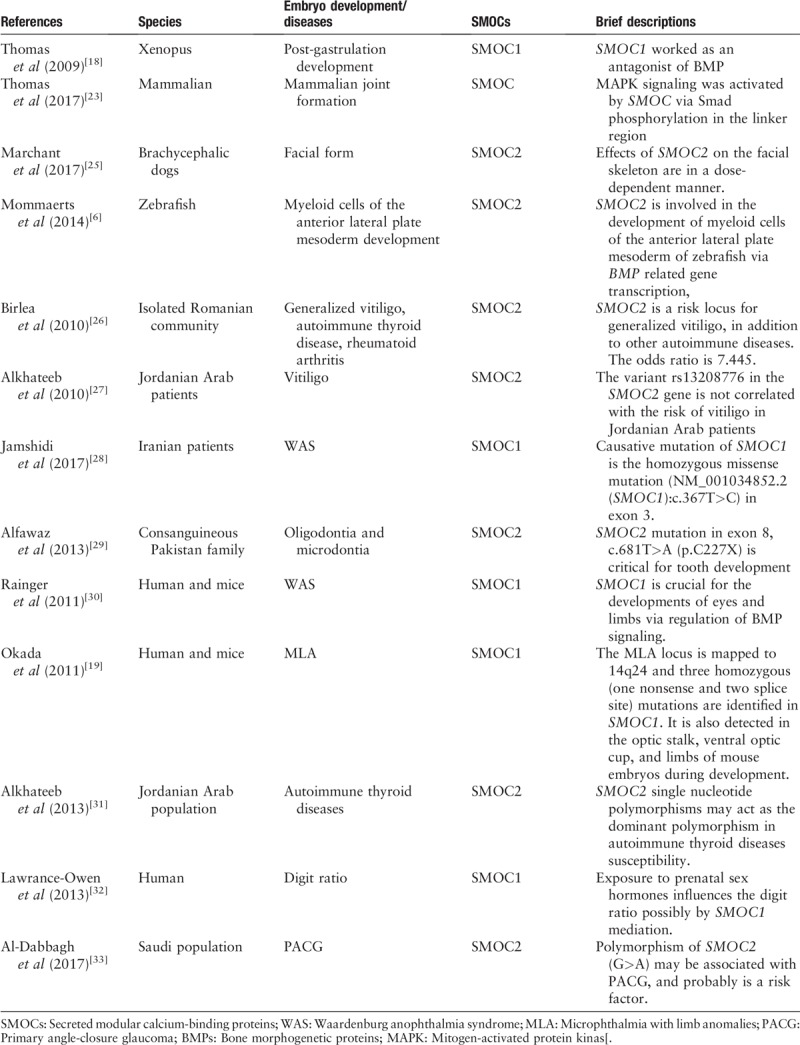
Molecularly, a previous study revealed that SMOCs function through the Smad (homologies to the Caenorhabditis elegans “small” worm phenotype and Drosophila mothers against decapentaplegic family of genes) signaling to activate mitogen-activated protein kinase (MAPK) signaling during the joint formation in the linker region.[23] Similar to the BMP family and its endogenous antagonists that regulate embryonic organ development,[18] SMOC1 can act as an antagonist of BMP signaling by mediating the MAPK-mediated Smad phosphorylation in the linker region.[18] In this regard, SMOC1 was not only a crucial factor to influence post-gastrulation development, but also regulated the formation of anatomic patterning, cell, or tissue fate specification through modulation of its related signaling.[18] Indeed, the C. elegans SMOC1 homolog acted as a positive modulator of the BMP signaling during development of C. elegans, and human SMOC1 and SMOC2 transgenes could each partially rescue phenotypes of SMOC1 mutants in worms, suggesting that SMOC1 modulation of BMP signaling was evolutionarily conserved.[24] The most recent findings of the function and molecular mechanisms of SMOCs action in embryo developments and related diseases are summarized in Table 1.
Table 1.
Role of SMOCs in embryo development and diseases.
Bone calcification
The extracellular matrix proteins (ECM), like SPARC, play a key role in the mineralization process of bone.[34] As a member of the SPARC protein family, SMOC1 is also highly expressed in the bone and the bone marrow mesenchymal stem cells.[35] Knockdown of SMOC1 expression inhibited osteoblast differentiation and mineralization, whereas SMOC1 overexpression enhanced osteoblast differentiation,[35] indicating that SMOC1 promotes osteoblast differentiation and mineralization. Furthermore, SMOC1 expression peaked on day 1 during post-natal induction of osteoblast differentiation, and gradually decreases in the subsequent 7 days. These studies suggest that SMOC1 positively regulates the early stage of osteoblast differentiation, but it may be not required for the late stage of osteoblast differentiation, similar to other ECM proteins.[35] Further investigation should focus on bettering our understanding of the underlying molecular mechanisms of SMOC1 action in calcification, such as SMOC1 in the regulation of receptor-mediating signaling.
SMOC2 was originally identified in the extracellular matrix of articular cartilage[5,36] and SMOC2 inhibited osteogenic differentiation and extracellular matrix mineralization through the SMOC2 EC domain.[11] Ectopic or excessive calcification contributes to diseases like chondrocalcinosis by calcium deposition in the skin or blood vessels. SMOC2 was able to downregulate the mineralization process in human umbilical vein endothelial cells, whereas SMOC-ΔEC, which lacks the EC domain, was unable to inhibit BMP2 signaling and SMOC-EC (the EC domain only)-induced activity of the BMP2 signaling.[23] However, knockdown of SMOC2 expression did not affect MC3T3-E1 cell calcification.[23] This implies that an increase in SMOC2 levels might result from cell mineralization, rather than as a cause. These findings are contradictory to a previous observation showing that SMOC2 was required for bone development.[37] One of the plausible reasons may be because MC3T3-E1 cells were derived from neonatal mouse calvaria and are already committed to calcification,[38] in which SMOC2 may have a less regulatory role.
Altogether, SMOCs are required for the tissue calcification process, although the regulatory functions of SMOC1 are different from SMOC2. To date, there are few publications that describe the role of other SMOC domains, especially how these unique domains participate in calcification and BMP-related signaling, which warrants further investigation.
Angiogenesis
SMOC1 possesses a pro-angiogenic activity and is a target of anti-angiogenic microRNA-223 (miR-223).[12] Knockdown of SMOC1 expression significantly attenuated the sprouting of endothelial cells in the aortic rings and delayed retinal angiogenesis in SMOC1+/− mice.[12] The inhibitory effect of SMOC1 silencing on angiogenesis was associated with upregulation of endoglin expression, which is an endothelium-specific type III auxiliary receptor in the transforming growth factor-β (TGF-β) receptor family and can induce Smad2 phosphorylation. Mouse endothelial cells were able to secrete SMOC1 protein to inhibit expression of the activin-like kinase (ALK) 5 and promote TGF-β signaling and ALK1 activation, resulting in endothelial cell proliferation and angiogenesis.[12] Furthermore, hypoxia can stimulate transcription of SMOC1 mRNA in human endothelial cells by downregulation of miR-223 expression.[12]SMOC2 overexpression acts in parallel synergize with vascular endothelial growth factor (VEGF) or basic fibroblast growth factor to stimulate DNA synthesis and the formation of endothelial cell network-like structures, whereas SMOC1 small interfering RNA (siRNA) treatment inhibited endothelial cell network formation and proliferation. These in vitro data were confirmed in mice with sub-dermal implantation of Matrigel plugs containing SMOC2 cDNA adenovirus.[39] Similarly, SMOC2 can promote cell migration, angiogenesis, and migration beyond the enhancement of the neointima formation.[8] Accordingly, SMOC2 may promote the pathological progression of endometriosis.[40]
Although there is high sequence similarity between SMOC and SPARC, these proteins have opposing effects on angiogenesis.[7] For example, SPARC was able to inhibit gastric cancer angiogenesis by attenuating expression of VEGF and matrix metalloproteinase 7.[41] The functional balance between the SMOCs and SPARC ultimately determines their effects on endothelial cell proliferation, angiogenesis, and other pathogenic processes. Further studies should focus on the molecular mechanisms by which SMOCs regulate angiogenesis.
In addition, a previous study reports that the smooth muscle associated protein 2 (SMAP2), originally isolated from human aortic 3V cDNA library, showed a similarity to SMOC2.[42] The SMAP2 protein contains two thyroglobulin type-1 (Tg1) domains, two EF-hand calcium-binding domains, and a putative signal sequence domain. The Tg1 domain can regulate proteolysis.[42] SMAP2 mRNA transcripts are detected predominantly in the aorta, skeletal muscle and heart, ovary, testis, stomach, small intestine and colon, thyroid gland, mammary gland, and prostate. SMAP2 expression was upregulated by 5.4 fold at 7 days post-surgery in a rat model of intraluminal balloon injury in the right carotid artery. Its expression was activated at day 3 and remained up to 14 days after surgery, suggesting that SMAP2 may be closely related to the proliferation and migration of vascular smooth muscle cell after vascular injury.
Cell cycle regulation
SPARC can directly bind to various growth factors, including the platelet-derived growth factor (PDGF),[43] VEGF,[44] TGF-β,[45] or fibroblast growth factor 2[46] to inhibit receptor-mediated signaling. In contrast, SPARC deficiency promoted the proliferation of mesangial cells, fibroblasts, and smooth muscle cells.[47] Similarly, high levels of SMOC2 expression were detected in the G1/S phase of the cell cycle in fibroblasts but these were reduced during the process of serum-stimulated cell cycle progression, suggesting that SMOC2 inhibits cell cycle progression and cell proliferation in fibroblasts.[7] In contrast, serum stimulation did not change SMOC1 expression in fibroblasts Swiss 3T3. These data suggest that both SMOC1 and SMOC2 have differing roles in the regulation of the cell cycle although the underlying molecular mechanisms are unclear. Furthermore, SMOC2 is required for growth factor-induced mitogenesis, especially for TGF-β.[7] SMOC2 ablation abrogated cyclin D1 levels in the G1 phase of the cell cycle in 3T3 cells.[7] Interestingly, SMOC2 ablation also reduced PDGF-induced DNA synthesis by 46% in cytomegalovirus-green fluorescent protein (GFP)-transfected cells, while SMOC2 ablation only decreases the PDGF-induced DNA synthesis by 4% in cyclin D1-overexpressing Swiss 3T3 cells.[7] These studies suggest that cyclin-D1-overexpressing cells had better responses to growth factor(s), independent of SMOC2. Actually, SMOC2 deficiency did not significantly affect the PDGF-mediated PDGFβR phosphorylation, regardless of PDGF addition,[7] or the PDGF-induced MAPK and protein kinase B (Akt) signaling and DNA synthesis.[7] Hence, SMOC2 may not be required for the PDGFβR signaling. SMOC2 is required for the effective activation of integrin-linked kinase (ILK). However, the precise mechanisms by which SMOC2 regulates ILK activation remain to be clarified. Defective ILK signaling due to the SMOC2 deficiency could retard the G1/S transition of the cell cycle and may be involved in SMOC2-regulated cyclin D1 expression.[7]
Cell attachment
The EC domain of SMOC proteins is an autonomously folding domain and functions to promote the adhesion of skin HaCaT cells in a heparin sulfate-dependent manner.[48] Downregulation of SMOC1 expression reduces endothelial cell adhesion to collagen I, laminin-III, and/or Matrigel.[12] The EC domain of SMOC1 contains unique basic amino acids, the glycosaminoglycan-binding site, which are not present in other SPARC family proteins.[48] However, heparin sulfate proteoglycans on the cell surface do not guarantee the successful binding of SMOCs to cell adhesion molecules because HaCaT cells do not adhere to the EC domain of SMOC1 in the presence of a chelating reagent, even if the recombinant EC domain can maintain such a heparin-binding activity.[48] SMOC2, unlike SMOC1, does not promote adhesion of HaCaT cells, although there are a high sequence similarity of heparin-binding sites between SMOC1 and SMOC2.[48] Maier et al[8] previously reported that SMOC2 was able to selectively promote the adhesion of epidermal cells, dependent on the EC domain of SMOC2 and the integrins αvβ6 and αvβ1, but did not affect fibroblast adhesion. Moreover, the EC domain of SMOC2 can induce cell migration and wound healing.[8] In basement membranes, there are many heparin sulfate proteoglycans, such as collagen XVIII and perlecan. There is a need for further investigation as to how SMOCs interact with them and regulate cell adhesion and migration.
Alteration of SMOCs in human diseases
Thus far, we reviewed and discussed SMOCs and their function in cells and tissues. Some studies have also revealed the alteration of SMOC1 and SMOC2 functions in the pathogenesis and progression of human diseases. Generally, alterations of SMOC1 and SMOC2 can occur at the genetic, transcriptional, and post-translational levels and the aberrant SMOC1 and SMOC2 expression can lead to the development of human diseases. We discuss them in more detail below.
Birth defects
SMOC1 homozygosity mapping and subsequent targeted mutation analysis revealed that SMOC1 mutations were associated with ophthalmo-acromelic syndrome (OAS), also known as Waardenburg Anophthalmia syndrome. OAS is defined by a combination of eye malformations, most commonly bilateral anophthalmia, with post-axial oligosyndactyly.[66] SMOC1 mutations include nonsense, frame-shift, and missense mutations. The latter mutations occurred in the second Tg1 domain of the protein, while the targeted pre-conditional gene-trap mutation of Smoc1 [Smoc1(tm1a)] results in a reduction of SMOC1 mRNA levels to 10% of the wild-type (WT) SMOC1 levels.[66] A recent study also reported a novel homozygous missense SMOC1 mutation (c.812G>A; p.Cys271Tyr) in a family of patients with Waardenburg anophthalmia syndrome.[49]
Tissue inflammation and fibrosis
Persistent chronic inflammation can lead to tissue fibrosis. A previous study showed that treatment of rat mesangial cells with interleukin 1 beta induced nitric oxide (NO) production, but reduced SMOC1 expression in rat mesangial cells.[50] The blockade of the inducible nitric oxide synthases activity upregulates SMOC1 expression to enhance rat glomerular inflammation, which is accompanied by an increase in the deposition of fibrin in the glomeruli in a rat model of anti-Thy1.1-induced chronic glomerulonephritis.[51] Furthermore, knockdown of SMOC1 expression using SMOC1 siRNA decreased TGF-β expression and activity of the TGF-β signaling, which in turn reduced Smad activation and its target gene expression.[51] Interestingly, NO was able to inhibit SMOC1 expression and attenuate TGF-β-mediated signaling.[51] However, the specific mechanisms underlying the regulation of NO on SMOC1 expression remains unclear. SMOC2 has similar biological effects on the pathological process of fibrosis. For example, Gerarduzzi et al[9] demonstrated that SMOC2 is a key regulator of the pathological secretome in a damaged kidney and that SMOC2 promoted kidney fibrosis. In contrast, neutralization of SMOC2 attenuated the TGF-β-induced fibrosis in NIH 3T3 cells.[9] Intriguingly, SMOC2 overexpression was able to induce mouse kidney fibrosis and deteriorated inflammation-induced kidney fibrosis.[9]
Bleomycin, a cytotoxic anti-cancer agent, can inhibit DNA synthesis but induce reactive oxygen species production, which may also cause pulmonary fibrosis.[10] A recent study showed that reduction of SMOC2 expression was able to attenuate bleomycin-induced pulmonary fibrosis through inhibition of TGF-β signaling.[8] Moreover, SMOC2 suppression also attenuated the nuclear factor kappa-B (NF-κB) signaling and inflammatory responses.[10] Besides, SMOC2 deficiency can ablate the bleomycin-induced macrophage activation and neutrophil infiltration in vivo and in vitro.[10] The pathological score, collagen accumulation, and pulmonary fibrosis degrees were all lower in SMOC2−/− mice than in WT mice after bleomycin treatment.[10] Similarly, the levels of NF-κB phosphorylation and TGF-β1 expression in the lung were also significantly lower in the SMOC2−/− mice than in SMOC2 WT mice.[10] These data suggest that SMOC2 participates in the pathogenic process of pulmonary fibrosis and that further study could lead to the use of SMOC2 as a potential therapeutic target to treat pulmonary fibrosis. Also, SMOC2 overexpression exacerbated the fibrotic process, whereas knockdown of SMOC2 expression attenuated TGF-β1-mediated fibrosis.[10] These data indicate that SMOC2 could be a potential regulator of TGF-β1 signaling and fibrosis.
However, a previous study of indomethacin and retinoic acid (RA) in the modification of mouse intestinal inflammation and fibrosis revealed that indomethacin/2,4,6-trinitrobenzene sulfonic acid (TNBS) enhanced and RA reduced inflammation, tissue destruction, and fibrosis in a mouse model of TNBS-induced intestinal fibrosis, in which SPARC expression was inversely associated with fibrosis, but not with inflammation.[52] This study may indicate the differential role of SMOCs vs. SPARC in the regulation of tissue fibrosis.
Cancer development and progression
It is well known that interactions between malignant cells and the extracellular milieu are critical for cancer development and progression.[53] SPARC, as a set of ECM proteins, can affect stromal and desmoplastic responses to tumor cells.[54] The role of SPARC in the regulation of ECM production could make it a candidate conditioner of tumors.[54] Consistent with this speculation, SPARC deficiency promoted tumor growth in mice,[55] and SMOCs also regulate malignant cell proliferation.[56] At the chromosome level, the SMOC2 gene region contains several tumor suppressor genes.[57,58] Previous studies revealed that SMOC2 expression was downregulated in gall bladder carcinoma[59] and advanced breast cancer.[60] In contrast, levels of SMOC2 mRNA were upregulated by 36.55 fold in metastatic head and neck squamous cell carcinoma tissues.[61] SMOC2 modulated keratinocyte adhesion through binding to integrins[8] and the latter is important in the regulation of cell anoikis resistance and maintenance of cancer stem cell phenotypes.[62,63] By targeting a cancer stem cell signature gene, SMOC2 was able to overcome chemoresistance and inhibit proliferation of endometrial carcinoma cells.[64] Furthermore, SMOC1 was thought to be a cancer-related protein related to tenascin-C expression, which is an ECM protein and is highly expressed in a large variety of human cancers.[65] SMOC1 expression was upregulated in cancer tissues, such as oligodendrogliomas and astrocytic tumors.[66] SMOC1 was able to inhibit tenascin-C-induced migration of glioma U87 cells.[65] In this section, we further discuss the role of SMOCs in the regulation of development and progression of hepatocellular carcinoma (HCC), colorectal cancer, and lung adenocarcinoma.
Hepatocellular carcinoma
To date, the role of SMOC2 on HCC development and progression is controversial. For instance, SMOC2 expression was significantly upregulated in HCC tissues and upregulated SMOC2 expression induced expression of the MAPK/extracellular regulated protein kinases and AKT signaling.[14] Moreover, upregulated SMOC2 expression was able to promote cell cycle progression and tumor cell proliferation through an increase in cyclin D1 expression.[7] Hence, SMOC2 may be further studied as a potential therapeutic target to treat HCC clinically. In contrast, Huang et al[13] reported that SMOC2 expression was downregulated in HCC tissues and SMOC2 expression was associated with a favorable overall survival and disease-free survival of patients with HCC. The further experimental study indicated that SMOC2 overexpression suppressed HCC cell migration, proliferation, and cell cycle progression.[13] Thus, future study is needed to clarify the role of SMOC2 in HCC; however, to date, there is no single study of SMOC1 in HCC reported in the literature.
Colorectal cancer
SMOCs were shown to be colorectal cancer biomarkers and potential therapeutic targets for colorectal cancer patients.[67] For example, recent studies revealed that high levels of SMOC2 and leucine-rich repeat containing G protein-coupled receptor 5 (Lgr5) expression were observed in human colorectal cancer tissues[68] and that SMOC2 overexpression was associated with the potent metastatic capacity of Lgr5-upregulated colorectal cancer cells.[67] Elevated SMOC2 expression was detected in the invasive front of tumor tissues and SMOC2 expression is indispensable for L1-mediated invasion of colorectal cancer.[68] In parallel, SMOC2 overexpression was able to induce colorectal cancer motility and liver metastasis and the oncogenic effect of SMOC2 was associated with modulation of ILK activity, whereas treatment of colorectal cancer cells with QLT0267, a selective p38 MAPK inhibitor or knockdown of ILK expression reduced the SMOC2-mediated oncogenic effects.[68]
The “serrated neoplasia pathway” was considered a route of colorectal cancer development,[69] and sessile serrated adenoma/hyperplastic polyps and traditional serrated adenomas (TSAs) are different premalignant lesions in colorectal cancer development. Methylation of the SMOC1 gene gradually increased during TSA development, but rarely occurred in sessile serrated adenoma/hyperplastic polyps.[69] Consistent with the elevated SMOC1 gene methylation, SMOC1 expression was downregulated in TSAs, but not in sessile serrated adenoma/hyperplastic polyps.[70] Therefore, SMOC1 might serve as a diagnostic marker for serrated lesions.
Lung adenocarcinoma
Lung adenocarcinoma is one of the most commonly diagnosed malignancy in the world and lung cancer metastasis usually leads to a poor prognosis.[71] Currently, the molecular mechanisms underlying lung cancer metastasis remain to be defined. SMOC2 has been identified as a pro-metastatic matricellular protein and knockdown of SMOC2 expression inhibited lung cancer metastasis.[72] Brady et al[72] showed that expression of Aryl hydrocarbon receptor nuclear translocator-like 2 (Arntl2), a paralog of the circadian transcription factor Arntl,[73] was upregulated in metastatic lung adenocarcinoma and associated with poor prognosis. Another study revealed that the pro-metastatic effects of Arntl2 are attributed to a ten-fold increase in SMOC2 expression and that knockdown of Arntl2 expression significantly reduced SMOC2 expression in metastatic lung cancer cells.[72] Their additional data showed that both Arntl2 and Clock were able to bind to the proximal SMOC2 gene promoter, implying that both Arntl2 and Clock can directly regulate SMOC2 expression in metastatic lung adenocarcinoma.[74,75] Given that there are multiple extracellular proteins involved in metastasis of different human cancers,[74,75] further studies are needed to better understand how these genes interact with SMOCs to impact lung cancer metastasis.
Conclusions
SMOC1 and SMOC2 are ECM proteins of the SPARC family with differential and unique characteristics in tissue distribution and cell functions. SMOC1 was necessary for the early stage of osteoblast differentiation, while SMOC2 inhibited osteoblast differentiation.[11,35] SMOC proteins were able to promote angiogenesis, fibrosis, and cell motility and adhesion. The basic SMOCs functions and signaling are summarized in Figure 1. It should be noted that the pro-angiogenic effects of SMOCs oppose those of SPARC and the imbalance between the SMOCs and SPARC might determine the outcome and potential pathological changes during disease.[7] Thus, although the EC domain of SMOCs can regulate the mineralization process and cell attachment, functions of other SMOC protein domains, especially their unique domains, require further investigation. Furthermore, the regulatory effect of SMOC2 on cell adhesion depends on the context of individual cell types.[48] The mechanisms by which SMOCs as the soluble matrix proteins enter into their targeted cells and bind to their related receptor(s) to regulate cell signaling remain unstudied. Besides, the molecular mechanisms underlying how SMOCs regulate the cell cycle, interact with growth factors, and contribute to cancer development and progression as well as regulate embryonic development should be clarified in future research.
Figure 1.
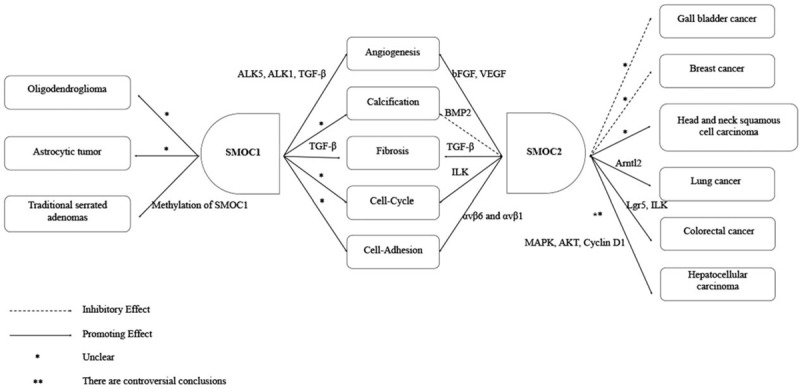
Illustration of basic SMOC functions in cells. Both SMOC1 and SMOC2 proteins are differentially expressed in various cells and tissues, which also function differently for embryonic development, homeostasis, and disease processes, that is, both SMOC1 and SMOC2 can promote angiogenesis, fibrosis, cell cycle progression, and cell adhesion. Molecularly, SMOC1 was able to regulate ALK5, ALK1, TGF-β, whereas SMOC2 regulated bFGF, VEGF in the angiogenesis process, although both SMOC1 and SMOC2 could regulate TGF-β in tissue fibrosis. SMOC2 can also regulate ILK in cell-cycle progression and αvβ6 and αvβ1 in the cell adhesion process, whereas the molecular mechanisms of SMOC1 in these two processes are not currently clear. SMOC1 enhanced calcification, but the underlying molecular mechanism remains to be defined. SMOC2 was able to inhibit calcification via the BMP2 signaling. Furthermore, SMOC1 deteriorated progression of oligodendrogliomas, astrocytic tumors, and traditional serrated adenoma, while SMOC2 is a risk factor for the development of head and neck squamous cell carcinoma, lung cancer, and colorectal cancer. SMOC2 can also act as a tumor suppressor in gallbladder and breast cancers. The potential mechanism involved in traditional serrated adenoma development was due to SMOC1 methylation, but the molecular mechanisms of SMOC1 involved in oligodendroglioma and astrocytic tumor are unclear. SMOC2 might regulate Arntl2 in lung cancer, Lgr5, and ILK in colorectal cancer development and interact with MAPK, AKT, and Cyclin D1 in HCC, but the role of SMOC2 in HCC development remains controversial. The potential mechanisms of SMOC2 involved in gallbladder and breast cancers as well as head and neck squamous cell carcinoma remains unknown. ALK: Activin-like kinase; Akt: protein kinase B; Arntl2: Aryl hydrocarbon receptor nuclear translocator-like 2; bFGF: Basic fibroblast growth factor; BMP: Bone morphogenetic protein; HCC: Hepatocellular carcinoma; ILK: Integrin-linked kinase; Lgr5: Leucine-rich repeat containing G protein-coupled receptor 5; MAPK: Mitogen-activated protein kinase; SMOC: Secreted modular calcium-binding protein; TGF-β: Transforming growth factor- β; VEGF: Vascular endothelial growth factor.
Funding
This study was supported in part by grants from the National Key Research and Development Program (No. 2018YFC1002600), the Science and Technology Planning Project of Guangdong Province, China (No. 2017A070701013, No. 2017030314109, and No. 2019B020230003), and the Guangdong Peak Project (No. DFJH201802).
Conflicts of interest
None.
Footnotes
How to cite this article: Gao Q, Mok HP, Zhuang J. Secreted modular calcium-binding proteins in pathophysiological processes and embryonic development. Chin Med J 2019;00:00–00. doi: 10.1097/CM9.0000000000000472
Qiang Gao and Hsiao-Pei Mok contributed equally to this work.
References
- 1.Bornstein P, Sage EH. Matricellular proteins: extracellular modulators of cell function. Curr Opin Cell Biol 2002; 14:608–616.. doi: 10.1016/S0955-0674(02)00361-7. [DOI] [PubMed] [Google Scholar]
- 2.Brekken RA, Sage EH. SPARC, a matricellular protein: at the crossroads of cell-matrix communication. Matrix Biol 2001; 19:816–827.. doi: 10.1016/S0945-053X(00)00133-5. [DOI] [PubMed] [Google Scholar]
- 3.Martinek N, Shahab J, Sodek J, Ringuette M. Is SPARC an evolutionarily conserved collagen chaperone? J Dent Res 2007; 86:296–305.. doi: 10.1177/154405910708600402. [DOI] [PubMed] [Google Scholar]
- 4.Vannahme C, Smyth N, Miosge N, Gosling S, Frie C, Paulsson M, et al. Characterization of SMOC-1, a novel modular calcium-binding protein in basement membranes. J Biol Chem 2002; 277:37977–37986.. doi: 10.1074/jbc.M203830200. [DOI] [PubMed] [Google Scholar]
- 5.Vannahme C, Gosling S, Paulsson M, Maurer P, Hartmann U. Characterization of SMOC-2, a modular extracellular calcium-binding protein. Biochem J 2003; 373:805–814.. doi: 10.1042/bj20030532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mommaerts H, Esguerra CV, Hartmann U, Luyten FP, Tylzanowski P. Smoc2 modulates embryonic myelopoiesis during zebrafish development. Dev Dyn 2014; 243:1375–1390.. doi: 10.1002/dvdy.24164. [DOI] [PubMed] [Google Scholar]
- 7.Liu P, Lu J, Cardoso WV, Vaziri C. The SPARC-related factor SMOC-2 promotes growth factor-induced cyclin D1 expression and DNA synthesis via integrin-linked kinase. Mol Biol Cell 2008; 19:248–261.. doi: 10.1091/mbc.e07-05-0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maier S, Paulsson M, Hartmann U. The widely expressed extracellular matrix protein SMOC-2 promotes keratinocyte attachment and migration. Exp Cell Res 2008; 314:2477–2487.. doi: 10.1016/j.yexcr.2008.05.020. [DOI] [PubMed] [Google Scholar]
- 9.Gerarduzzi C, Kumar RK, Trivedi P, Ajay AK, Iyer A, Boswell S, et al. Silencing SMOC2 ameliorates kidney fibrosis by inhibiting fibroblast to myofibroblast transformation. JCI Insight 2017; 2:90299.doi: 10.1172/jci.insight.90299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luo L, Wang CC, Song XP, Wang HM, Zhou H, Sun Y, et al. Suppression of SMOC2 reduces bleomycin (BLM)-induced pulmonary fibrosis by inhibition of TGF-beta1/SMADs pathway. Biomed Pharmacother 2018; 105:841–847.. doi: 10.1016/j.biopha.2018.03.058. [DOI] [PubMed] [Google Scholar]
- 11.Peeters T, Monteagudo S, Tylzanowski P, Luyten FP, Lories R, Cailotto F. SMOC2 inhibits calcification of osteoprogenitor and endothelial cells. PLoS One 2018; 13:e0198104.doi: 10.1371/journal.pone.0198104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Awwad K, Hu J, Shi L, Mangels N, Abdel Malik R, Zippel N, et al. Role of secreted modular calcium-binding protein 1 (SMOC1) in transforming growth factor beta signalling and angiogenesis. Cardiovasc Res 2015; 106:284–294.. doi: 10.1093/cvr/cvv098. [DOI] [PubMed] [Google Scholar]
- 13.Huang XQ, Zhou ZQ, Zhang XF, Chen CL, Tang Y, Zhu Q, et al. Overexpression of SMOC2 attenuates the tumorigenicity of hepatocellular carcinoma cells and is associated with a positive postoperative prognosis in human hepatocellular carcinoma. J Cancer 2017; 8:3812–3827.. doi: 10.7150/jca.20775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Su JR, Kuai JH, Li YQ. Smoc2 potentiates proliferation of hepatocellular carcinoma cells via promotion of cell cycle progression. World J Gastroenterol 2016; 22:10053–10063.. doi: 10.3748/wjg.v22.i45.10053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brekken RA, Sage EH. SPARC, a matricellular protein: at the crossroads of cell-matrix. Matrix Biol 2000; 19:569–580.. doi: 10.1016/S0945-053X(00)00133-5. [DOI] [PubMed] [Google Scholar]
- 16.Novinec M, Kovacic L, Skrlj N, Turk V, Lenarcic B. Recombinant human SMOCs produced by in vitro refolding: calcium-binding properties and interactions with serum proteins. Protein Expr Purif 2008; 62:75–82.. doi: 10.1016/j.pep.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 17.Pazin DE, Albrecht KH. Developmental expression of Smoc1 and Smoc2 suggests potential roles in fetal gonad and reproductive tract differentiation. Dev Dyn 2009; 238:2877–2890.. doi: 10.1002/dvdy.22124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thomas JT, Canelos P, Luyten FP, Moos M., Jr Xenopus SMOC-1 Inhibits bone morphogenetic protein signaling downstream of receptor binding and is essential for postgastrulation development in Xenopus. J Biol Chem 2009; 284:18994–19005.. doi: 10.1074/jbc.M807759200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Okada I, Hamanoue H, Terada K, Tohma T, Megarbane A, Chouery E, et al. SMOC1 is essential for ocular and limb development in humans and mice. Am J Hum Genet 2011; 88:30–41.. doi: 10.1016/j.ajhg.2010.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gersdorff N, Muller M, Schall A, Miosge N. Secreted modular calcium-binding protein-1 localization during mouse embryogenesis. Histochem Cell Biol 2006; 126:705–712.. doi: 10.1007/s00418-006-0200-7. [DOI] [PubMed] [Google Scholar]
- 21.Srivastava J, Premi S, Kumar S, Parwez I, Ali S. Characterization of Smoc-1 uncovers two transcript variants showing differential tissue and age specific expression in Bubalus bubalis. BMC Genomics 2007; 8:436.doi: 10.1186/1471-2164-8-436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bloch-Zupan A, Jamet X, Etard C, Laugel V, Muller J, Geoffroy V, et al. Homozygosity mapping and candidate prioritization identify mutations, missed by whole-exome sequencing, in SMOC2, causing major dental developmental defects. Am J Hum Genet 2011; 89:773–781.. doi: 10.1016/j.ajhg.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thomas JT, Eric Dollins D, Andrykovich KR, Chu T, Stultz BG, Hursh DA, et al. SMOC can act as both an antagonist and an expander of BMP signaling. Elife 2017; 6:e17935.doi: 10.7554/eLife.17935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DeGroot MS, Shi H, Eastman A, McKillop AN, Liu J. The Caenorhabditis elegans SMOC-1 protein acts cell nonautonomously to promote bone morphogenetic protein signaling. Genetics 2019; 211:683–702.. doi: 10.1534/genetics.118.301805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marchant TW, Johnson EJ, McTeir L, Johnson CI, Gow A, Liuti T, et al. Canine brachycephaly is associated with a retrotransposon-mediated missplicing of SMOC2. Curr Biol 2017; 27:1573–1584.e6.. doi: 10.1016/j.cub.2017.04.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Birlea SA, Gowan K, Fain PR, Spritz RA. Genome-wide association study of generalized vitiligo in an isolated European founder population identifies SMOC2, in close proximity to IDDM8. J Invest Dermatol 2010; 130:798–803.. doi: 10.1038/jid.2009.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alkhateeb A, Al-Dain Marzouka N, Qarqaz F. SMOC2 gene variant and the risk of vitiligo in Jordanian Arabs. Eur J Dermatol 2010; 20:701–704.. doi: 10.1684/ejd.2010.1095. [DOI] [PubMed] [Google Scholar]
- 28.Jamshidi J, Abdollahi S, Ghaedi H, Alehabib E, Tafakhori A, Alinaghi S, et al. A novel mutation in SMOC1 and variable phenotypic expression in two patients with Waardenburg anophthalmia syndrome. Eur J Med Genet 2017; 60:578–582.. doi: 10.1016/j.ejmg.2017.08.006. [DOI] [PubMed] [Google Scholar]
- 29.Alfawaz S, Fong F, Plagnol V, Wong FS, Fearne J, Kelsell DP. Recessive oligodontia linked to a homozygous loss-of-function mutation in the SMOC2 gene. Arch Oral Biol 2013; 58:462–466.. doi: 10.1016/j.archoralbio.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 30.Rainger J, van Beusekom E, Ramsay JK, McKie L, Al-Gazali L, Pallotta R, et al. Loss of the BMP antagonist, SMOC-1, causes ophthalmo-acromelic (Waardenburg Anophthalmia) syndrome in humans and mice. PLoS Genet 2011; 7:e1002114.doi: 10.1371/journal.pgen.1002114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alkhateeb A, Marzouka NA, Tashtoush R. Variants in PTPN22 and SMOC2 genes and the risk of thyroid disease in the Jordanian Arab population. Endocrine 2013; 44:702–709.. doi: 10.1007/s12020-013-9908-z. [DOI] [PubMed] [Google Scholar]
- 32.Lawrance-Owen AJ, Bargary G, Bosten JM, Goodbourn PT, Hogg RE, Mollon JD. Genetic association suggests that SMOC1 mediates between prenatal sex hormones and digit ratio. Hum Genet 2013; 132:415–421.. doi: 10.1007/s00439-012-1259-y. [DOI] [PubMed] [Google Scholar]
- 33.Al-Dabbagh N, Al-Shahrani H, Al-Dohayan N, Mustafa M, Arfin M, Al-Asmari AK. The SPARC-related modular calcium binding protein 2 (SMOC2) gene polymorphism in primary glaucoma: a case-control study. Clin Ophthalmol 2017; 11:549–555.. doi: 10.2147/opth.s126459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Delany AM, Amling M, Priemel M, Howe C, Baron R, Canalis E. Osteopenia and decreased bone formation in osteonectin-deficient mice. J Clin Invest 2000; 105:915–923.. doi: 10.1172/jci7039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Choi YA, Lim J, Kim KM, Acharya B, Cho JY, Bae YC, et al. Secretome analysis of human BMSCs and identification of SMOC1 as an important ECM protein in osteoblast differentiation. J Proteome Res 2010; 9:2946–2956.. doi: 10.1021/pr901110q. [DOI] [PubMed] [Google Scholar]
- 36.Chang SC, Hoang B, Thomas JT, Vukicevic S, Luyten FP, Ryba NJ, et al. Cartilage-derived morphogenetic proteins. New members of the transforming growth factor-beta superfamily predominantly expressed in long bones during human embryonic development. J Biol Chem 1994; 269:28227–28234.. [PubMed] [Google Scholar]
- 37.Melvin VS, Feng W, Hernandez-Lagunas L, Artinger KB, Williams T. A morpholino-based screen to identify novel genes involved in craniofacial morphogenesis. Dev Dyn 2013; 242:817–831.. doi: 10.1002/dvdy.23969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sudo H, Kodama HA, Amagai Y, Yamamoto S, Kasai S. In vitro differentiation and calcification in a new clonal osteogenic cell line derived from newborn mouse calvaria. J Cell Biol 1983; 96:191–198.. doi: 10.1083/jcb.96.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rocnik EF, Liu P, Sato K, Walsh K, Vaziri C. The novel SPARC family member SMOC-2 potentiates angiogenic growth factor activity. J Biol Chem 2006; 281:22855–22864.. doi: 10.1074/jbc.M513463200. [DOI] [PubMed] [Google Scholar]
- 40.Araujo FM, Meola J, Rosa ESJC, Paz CCP, Ferriani RA, Nogueira AA. Increased expression of ID2, PRELP and SMOC2 genes in patients with endometriosis. Braz J Med Biol Res 2017; 50:e5782.doi: 10.1590/1414-431x20175782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang JL, Chen GW, Liu YC, Wang PY, Wang X, Wan YL, et al. Secreted protein acidic and rich in cysteine (SPARC) suppresses angiogenesis by down-regulating the expression of VEGF and MMP-7 in gastric cancer. PLoS One 2012; 7:e44618.doi: 10.1371/journal.pone.0044618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nishimoto S, Hamajima Y, Toda Y, Toyoda H, Kitamura K, Komurasaki T. Identification of a novel smooth muscle associated protein, smap2, upregulated during neointima formation in a rat carotid endarterectomy model. Biochim Biophys Acta 2002; 1576:225–230.. doi: 10.1016/S0167-4781(02)00345-7. [DOI] [PubMed] [Google Scholar]
- 43.Motamed K, Funk SE, Koyama H, Ross R, Raines EW, Sage EH. Inhibition of PDGF-stimulated and matrix-mediated proliferation of human vascular smooth muscle cells by SPARC is independent of changes in cell shape or cyclin-dependent kinase inhibitors. J Cell Biochem 2002; 84:759–771.. doi: 10.1002/jcb.10095. [DOI] [PubMed] [Google Scholar]
- 44.Kupprion C, Motamed K, Sage EH. SPARC (BM-40, osteonectin) inhibits the mitogenic effect of vascular endothelial growth factor on microvascular endothelial cells. J Biol Chem 1998; 273:29635–29640.. doi: 10.1074/jbc.273.45.2963. [DOI] [PubMed] [Google Scholar]
- 45.Francki A, McClure TD, Brekken RA, Motamed K, Murri C, Wang T, et al. SPARC regulates TGF-beta1-dependent signaling in primary glomerular mesangial cells. J Cell Biochem 2004; 91:915–925.. doi: 10.1002/jcb.20008. [DOI] [PubMed] [Google Scholar]
- 46.Motamed K, Blake DJ, Angello JC, Allen BL, Rapraeger AC, Hauschka SD, et al. Fibroblast growth factor receptor-1 mediates the inhibition of endothelial cell proliferation and the promotion of skeletal myoblast differentiation by SPARC: a role for protein kinase A. J Cell Biochem 2003; 90:408–423.. doi: 10.1002/jcb.10645. [DOI] [PubMed] [Google Scholar]
- 47.Bradshaw AD, Francki A, Motamed K, Howe C, Sage EH. Primary mesenchymal cells isolated from SPARC-null mice exhibit altered morphology and rates of proliferation. Mol Biol Cell 1999; 10:1569–1579.. doi: 10.1091/mbc.10.5.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Klemencic M, Novinec M, Maier S, Hartmann U, Lenarcic B. The heparin-binding activity of secreted modular calcium-binding protein 1 (SMOC-1) modulates its cell adhesion properties. PLoS One 2013; 8:e56839.doi: 10.1371/journal.pone.0056839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ullah A, Umair M, Ahmad F, Muhammad D, Basit S, Ahmad W. A novel homozygous variant in the SMOC1 gene underlying Waardenburg anophthalmia syndrome. Ophthalmic Genet 2017; 38:335–339.. doi: 10.1080/13816810.2016.1227456. [DOI] [PubMed] [Google Scholar]
- 50.Dreieicher E, Beck KF, Lazaroski S, Boosen M, Tsalastra-Greul W, Beck M, et al. Nitric oxide inhibits glomerular TGF-beta signaling via SMOC-1. J Am Soc Nephrol 2009; 20:1963–1974.. doi: 10.1681/asn.2008060653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Westenfeld R, Gawlik A, de Heer E, Kitahara M, Abou-Rebyeh F, Floege J, et al. Selective inhibition of inducible nitric oxide synthase enhances intraglomerular coagulation in chronic anti-Thy 1 nephritis. Kidney Int 2002; 61:834–838.. doi: 10.1046/j.1523-1755.2002.00229.x. [DOI] [PubMed] [Google Scholar]
- 52.Klopcic B, Appelbee A, Raye W, Lloyd F, Jooste JC, Forrest CH, et al. Indomethacin and retinoic acid modify mouse intestinal inflammation and fibrosis: a role for SPARC. Dig Dis Sci 2008; 53:1553–1563.. doi: 10.1007/s10620-007-0068-y. [DOI] [PubMed] [Google Scholar]
- 53.Rangarajan A, Weinberg RA. Opinion: comparative biology of mouse versus human cells: modelling human cancer in mice. Nat Rev Cancer 2003; 3:952–959.. doi: 10.1038/nrc1235. [DOI] [PubMed] [Google Scholar]
- 54.Framson PE, Sage EH. SPARC and tumor growth: where the seed meets the soil? J Cell Biochem 2004; 92:679–690.. doi: 10.1002/jcb.20091. [DOI] [PubMed] [Google Scholar]
- 55.Brekken RA, Puolakkainen P, Graves DC, Workman G, Lubkin SR, Sage EH. Enhanced growth of tumors in SPARC null mice is associated with changes in the ECM. J Clin Invest 2003; 111:487–495.. doi: 10.1172/jci16804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Torrente A, Lukk M, Xue V, Parkinson H, Rung J, Brazma A. Identification of cancer related genes using a comprehensive map of human gene expression. PLoS One 2016; 11:e0157484.doi: 10.1371/journal.pone.0157484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Steinemann D, Gesk S, Zhang Y, Harder L, Pilarsky C, Hinzmann B, et al. Identification of candidate tumor-suppressor genes in 6q27 by combined deletion mapping and electronic expression profiling in lymphoid neoplasms. Genes Chromosomes Cancer 2003; 37:421–426.. doi: 10.1002/gcc.10231. [DOI] [PubMed] [Google Scholar]
- 58.Bignone PA, Lee KY, Liu Y, Emilion G, Finch J, Soosay AE, et al. RPS6KA2, a putative tumour suppressor gene at 6q27 in sporadic epithelial ovarian cancer. Oncogene 2007; 26:683–700.. doi: 10.1038/sj.onc.1209827. [DOI] [PubMed] [Google Scholar]
- 59.Gu X, Li B, Jiang M, Fang M, Ji J, Wang A, et al. RNA sequencing reveals differentially expressed genes as potential diagnostic and prognostic indicators of gallbladder carcinoma. Oncotarget 2015; 6:20661–20671.. doi: 10.18632/oncotarget.3861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fidalgo F, Rodrigues TC, Pinilla M, Silva AG, Maciel Mdo S, Rosenberg C, et al. Lymphovascular invasion and histologic grade are associated with specific genomic profiles in invasive carcinomas of the breast. Tumour Biol 2015; 36:1835–1848.. doi: 10.1007/s13277-014-2786-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hyakusoku H, Sano D, Takahashi H, Hatano T, Isono Y, Shimada S, et al. JunB promotes cell invasion, migration and distant metastasis of head and neck squamous cell carcinoma. J Exp Clin Cancer Res 2016; 35:6.doi: 10.1186/s13046-016-0284-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Desgrosellier JS, Cheresh DA. Integrins in cancer: biological implications and therapeutic opportunities. Nat Rev Cancer 2010; 10:9–22.. doi: 10.1038/nrc2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Prowse AB, Chong F, Gray PP, Munro TP. Stem cell integrins: implications for ex-vivo culture and cellular therapies. Stem Cell Res 2011; 6:1–12.. doi: 10.1016/j.scr.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 64.Lu H, Ju DD, Yang GD, Zhu LY, Yang XM, Li J, et al. Targeting cancer stem cell signature gene SMOC-2 overcomes chemoresistance and inhibits cell proliferation of endometrial carcinoma. EBioMedicine 2019; 40:276–289.. doi: 10.1016/j.ebiom.2018.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Brellier F, Ruggiero S, Zwolanek D, Martina E, Hess D, Brown-Luedi M, et al. SMOC1 is a tenascin-C interacting protein over-expressed in brain tumors. Matrix Biol 2011; 30:225–233.. doi: 10.1016/j.matbio.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 66.Boon K, Edwards JB, Eberhart CG, Riggins GJ. Identification of astrocytoma associated genes including cell surface markers. BMC Cancer 2004; 4:39.doi: 10.1186/1471-2407-4-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shvab A, Haase G, Ben-Shmuel A, Gavert N, Brabletz T, Dedhar S, et al. Induction of the intestinal stem cell signature gene SMOC-2 is required for L1-mediated colon cancer progression. Oncogene 2016; 35:549–557.. doi: 10.1038/onc.2015.127. [DOI] [PubMed] [Google Scholar]
- 68.Merlos-Suarez A, Barriga FM, Jung P, Iglesias M, Cespedes MV, Rossell D, et al. The intestinal stem cell signature identifies colorectal cancer stem cells and predicts disease relapse. Cell Stem Cell 2011; 8:511–524.. doi: 10.1016/j.stem.2011.02.020. [DOI] [PubMed] [Google Scholar]
- 69.Leggett B, Whitehall V. Role of the serrated pathway in colorectal cancer pathogenesis. Gastroenterology 2010; 138:2088–2100.. doi: 10.1053/j.gastro.2009.12.066. [DOI] [PubMed] [Google Scholar]
- 70.Aoki H, Yamamoto E, Takasawa A, Niinuma T, Yamano HO, Harada T, et al. Epigenetic silencing of SMOC1 in traditional serrated adenoma and colorectal cancer. Oncotarget 2018; 9:4707–4721.. doi: 10.18632/oncotarget.23523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.de Castro J, Rodriguez MC, Martinez-Zorzano VS, Sanchez-Rodriguez P, Sanchez-Yague J. Erythrocyte fatty acids as potential biomarkers in the diagnosis of advanced lung adenocarcinoma, lung squamous cell carcinoma, and small cell lung cancer. Am J Clin Pathol 2014; 142:111–120.. doi: 10.1309/ajcp1quqqllt8bli. [DOI] [PubMed] [Google Scholar]
- 72.Brady JJ, Chuang CH, Greenside PG, Rogers ZN, Murray CW, Caswell DR, et al. An Arntl2-driven secretome enables lung adenocarcinoma metastatic self-sufficiency. Cancer Cell 2016; 29:697–710.. doi: 10.1016/j.ccell.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hogenesch JB, Gu YZ, Moran SM, Shimomura K, Radcliffe LA, Takahashi JS, et al. The basic helix-loop-helix-PAS protein MOP9 is a brain-specific heterodimeric partner of circadian and hypoxia factors. J Neurosci 2000; 20:Rc83.doi: 10.1523/JNEUROSCI.20-13-j0002.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhou SL, Dai Z, Zhou ZJ, Chen Q, Wang Z, Xiao YS, et al. CXCL5 contributes to tumor metastasis and recurrence of intrahepatic cholangiocarcinoma by recruiting infiltrative intratumoral neutrophils. Carcinogenesis 2014; 35:597–605.. doi: 10.1093/carcin/bgt397. [DOI] [PubMed] [Google Scholar]
- 75.Bo H, Zhang S, Gao L, Chen Y, Zhang J, Chang X, et al. Upregulation of Wnt5a promotes epithelial-to-mesenchymal transition and metastasis of pancreatic cancer cells. BMC Cancer 2013; 13:496.doi: 10.1186/1471-2407-13-496. [DOI] [PMC free article] [PubMed] [Google Scholar]


