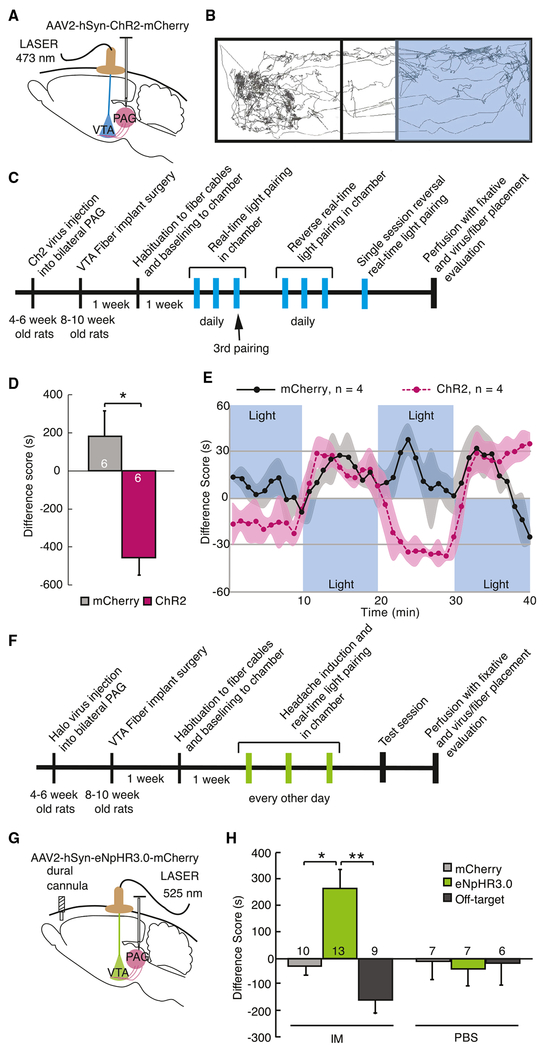
Figure 4. Activation of vlPAG-to-VTA Afferents Is Aversive and Required for Headache Aversiveness.
(A) Schematic of surgical preparation using ChR2 to selectively activate vlPAG axon terminals in the VTA in 3 replicate groups. Control animals were injected with sham virus, AAV2-hSyn-mCherry. 6-8 weeks later, optical fibers were implanted, bilaterally aimed at the VTA. Animals showed no chamber bias at baseline.
(B) Example track tracing of an animal during testing in which blue light (473 nm, 20 Hz, 5 ms pulse, 10-12 mW) commenced when the rat entered the right side of the chamber and was turned off when animal exited that side of the chamber.
(C) Timeline of the real-time optical stimulation place pairing protocol. Animals were placed in the test chamber for 20-min sessions daily in which blue light stimulation was paired with one side of the chamber. After 3 sessions with light paired with one side of the chamber, light was then paired to the opposite chamber for 3 daily 20-min sessions for reversal training. After 6 sessions, 4 animals underwent a 40-min session in which light pairing was reversed every 10 min.
(D) During the 3rd training session, animals with active ChR2 virus infection avoided the chamber with light stimulation compared with animals injected with sham virus. The difference score is calculated as the time spent in the stimulation-paired chamber minus the time spent in the nostimulation chamber (*p < 0.05). Plot represents mean ± SEM.
(E) Averaged real-time difference score from 4 animals during the last session, with stimulation alternating between chambers every 10 min.
(F) Timeline of the real-time optical inhibition place pairing protocol. Animals from 5 replicate groups received either intradural IMs or PBS 5 min before being placed into the test chamber. After 3 daily 20-min sessions of green light pairing (525 nm, 16-18 mW) with one side of the chamber, animals were tested the following day in the chamber without light inhibition.
(G) Schematic of surgical preparation for the behavioral experiment using halorhodopsin to selectively silence vlPAG inputs to the VTA after dural IMs to induce headache. Sham virus or AAV2-hSyn-eNpHR3.0-mCherry was injected into the vlPAG. Bilateral optical fibers were implanted directed to the VTA 6-8 weeks later.
(H) Difference scores were measured during the testing session with no stimulation (*p < 0.05, **p < 0.01), demonstrating that inactivation of the vlPAG-to-VTA connection was appetitive only in rats that received IMs, suggesting it relievesthe aversive state induced by dural IMs. Plot represents mean ± SEM. See also Figure S4.