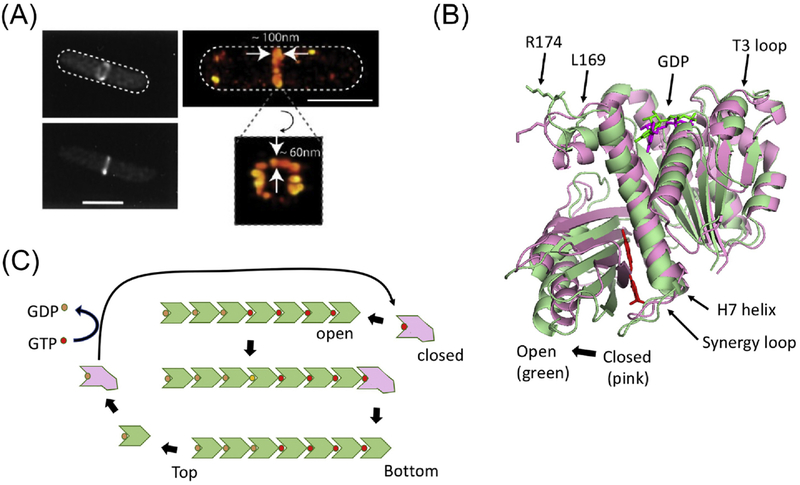
Figure 1. Cooperative assembly of FtsZ.
(A) Conventional and super resolution images of the Z ring (from ref. 14). (B) The two conformations of FtsZ. The open (green – PDB:3VOB) and closed (pink-PDB:2RHL) forms of FtsZ (from Staphylococcus aureus) are superimposed. The PC190723 inhibitor (red) and is present in the structure of the open form. Residues corresponding to R174 and L169 are indicated as are other important features. (C) A model for the cooperative assembly of FtsZ. In this model FtsZ in the closed form adds to the bottom end (the kinetic plus end) of a filament and switches conformation to the open form. Subsequent GTP hydrolysis leads to weakening of longitudinal bonds and loss of subunits from the minus end. Such subunits revert to the closed form and undergo nucleotide exchange before adding to the growing end.