Supplemental Digital Content is available in the text
Keywords: breakpoint, genetic counseling, male infertility, non-Robertsonian translocation
Abstract
For genetic counseling of male carriers of chromosomal translocations, the specific chromosomes and breakpoints involved in the translocation are relevant to know. The structural chromosomal abnormalities may lead to abnormal sperm counts, infertility, and miscarriage. These are related to the specific chromosomes and breakpoints involved in the translocation. To date, over 200 cases of non-Robertsonian translocation in male carriers have been described that involve chromosomes 13, 14, or 15.
This study reports of 28 male carriers from our clinic with balanced reciprocal translocations of chromosome 13, 14, or 15, and a literature review of 201 cases. The 28 male carriers from our clinic were diagnosed by cytogenetic analyses: 19 subjects suffered from pregestational infertility and 9 from gestational infertility. The most common translocations were t(7;13), t(10;14), and t(3;15), observed respectively in 13 (46%), 8 (29%), and 8 (29%) of our subjects. The literature cases (n = 201) involved chromosome 13 (n = 83, 41%), chromosome 14 (n = 56, 28%) or 15 (n = 62, 31%) in which 75 breakpoints were identified, the most common breakpoint, 13q22, was observed in 12 subjects (6%), followed by 14q32 (n = 11), 15q15 (n = 9), and 15q22 (n = 9). Most breakpoints were related to gestational infertility, while breakpoints at 13p13, 13p12, 13p11.2, 13p11, 13q11, 13q15, 14p12, 14p10, 15p13, 15p10, and 15q22.2 were associated with pregestational infertility.
Carriers of non-Robertsonian translocations involving chromosome 13, 14, or 15 and experiencing infertility should receive counseling with regard to chromosomal breakpoints as there seem to be consequences for treatment. Intracytoplasmic sperm injection with preimplantation genetic diagnosis (PGD) for the carriers with oligozoospermia, microscopic testicular sperm extraction or sperm from the sperm bank for the carriers with azoospermia should be considered for pregestational infertility. The carriers with gestational infertility can choose PGD or prenatal diagnosis
1. Introduction
Chromosomal abnormalities play a major role in male infertility as structural chromosomal aberrations are up to 10 times more common.[1,2] Karyotype analysis is therefore relevant in the work-up of infertility.[3] The structural chromosomal abnormalities may lead to abnormal sperm counts, infertility, and miscarriage.[4,5] Robertsonian translocation is one of the most common structural chromosomal abnormalities, and involves group D (chromosomes 13, 14, 15) or G chromosomes (chromosomes 21, 22). Previous research has shown that carriers of Robertsonian translocation exhibit azoospermia because of changes in interchromosomal effect or show an increased frequency of disomic and diploid spermatocytes.[6,7] However, reports of non-Robertson (balanced) translocations involving group D chromosome are rare.
Carriers of balanced translocations are phenotypically not to be recognized; however, they may suffer infertility or spontaneous abortions.[8] These are related to the specific chromosomes and breakpoints involved in the translocation.[9] Previous reports indicate that the involvement of group D chromosomes in non-Robertson translocation is related to male infertility. Mikelsaar et al[10] further a reported an infertility case with balanced reciprocal translocation t(5;13)(q33;q12.1) and a microduplication in the region 9q31.1; they hypothesize that haploinsufficiency of the TUBA3C (tubulin alpha 3c) gene could cause the sperm immobility and abnormal sperm morphology as observed in this case. Jiang et al[11] reported oligospermia in a carrier of the reciprocal translocation of t(8;15) and identified an association between chromosomal behavior and apoptosis of primary spermatocytes. In addition, the KATNAL1 gene that plays a role in the regulation of Sertoli cell microtubule dynamics has been mapped on chromosome 13q12.3, its role in spermiogenesis is indispensable.[12] Previous studies have shown that the chromosome 15q15.3 region harbors CATSPER2, STRC, and PPIP5K1 genes, all associated with severely impaired spermatogenesis,[13] while spermatogenesis-associated protein 8 (SPATA8), a testis-specific gene, has been mapped to chromosome 15q26.2.[14] If translocation breakpoints interrupt these vital gene structures, then it is highly likely that the patients involved will suffer infertility.
The aim of this study is to explore the association between the clinical characteristics of male infertility in carriers of non-Robertsonian translocations involving the chromosomes 13, 14, or 15, with regard to the provision of appropriate genetic counseling.
2. Subjects and methods
2.1. Subjects and study design
We performed a single-centre retrospective study of subjects with non-Robertsonian translocations in chromosome 13, 14, or 15 in infertile men, and searched the literature using the PubMed database using the search terms, which is “chromosome/translocation/sperm” and “chromosome/translocation/abortion” on June 1 to 15, 2018.
The study was approved by the Ethics Committee of the First Hospital of Jilin University. Between July 2010 and December 2015, 28 men suffering from infertility were recruited from the outpatients department of the Centre for Reproductive Medicine at the First Hospital of Jilin University, Changchun, China. All subjects underwent a physical examination and a semen analysis, and completed a detailed questionnaire on smoking (tobacco), drinking (alcohol), marital status, childbearing history, spontaneous abortion, medical history, and working conditions. According to our previously published classification of smoking and drinking,[15] all questionnaires included smoking profile (733 [14%] heavy smokers, 1937 [37%] moderate, 2251 [43%] mild, and 314 [6] nonsmokers), and alcohol drinking profile (heavy drinking 157 [3%], moderate 942 [18%], mild 3507 [67%], and no alcohol drinking 629 [12%]). Azoospermia and oligozoospermia were defined by criteria described previously.[16]
2.2. Cytogenetic analysis
From each subject, we carried out a karyotype analysis of peripheral blood lymphocytes: peripheral blood (0.5 mL) was cultured in sterile tubes containing 30 U/mL heparin for 72 hours (Culture media, Yishengjun; Guangzhou Baidi Biotech, Guangzhou, China) and subsequently treated with 20 μg/mL colcemid for 1 hour. G-banding of metaphase chromosomes and karyotype analysis were performed as in our previous study.[16]
2.3. Translocation breakpoints
We used PubMed to carry out a literature search for non-Robertsonian translocations involving chromosomes 13, 14, or 15 in association with male infertility. We excluded translocations involving chromosome 13, 14, or 15, without reported breakpoints (n = 3). We analyzed the relationship between translocation breakpoints and male infertility and miscarriage.
3. Results
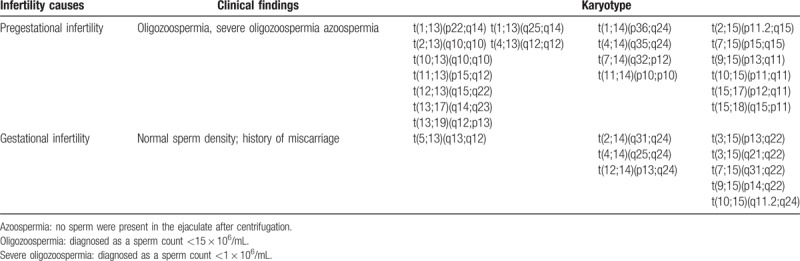
We identified in our 28 cases with non-Robertsonian translocations involvement of chromosomes 13 (n = 10), 14 (n = 7), or 15 (n = 11). Nineteen subjects with pregestational infertility (the main characteristic being azoospermia or oligozoospermia), the remaining nine subjects exhibited gestational infertility (the main finding being normal semen parameters, in which the patient's partner conceived but tended to miscarry). The karyotype analyses of these 28 subjects in relation to chromosome 13, 14, or 15 translocations are summarized in Table 1.
Table 1.
Karyotypes of non-Robertsonian translocation involving group D chromosomes and their clinical features.
Our literature searches identified 201 carriers of non-Robertsonian translocation, 83 subjects in chromosome 13, 56 subjects in chromosome 14 and 62 in chromosome 15. The karyotypes of, and breakpoints in, group D chromosomes, and their related clinical symptoms, are summarized in a supplementary files (Table 1). The most common translocations are t(7;13), t(10;14), and t(3;15), observed respectively in 13, 8, and 8 subjects. In male infertility, the distribution of other chromosomes involved in the translocation with chromosome 13, 14, or 15 are shown in Figure 1.
Figure 1.
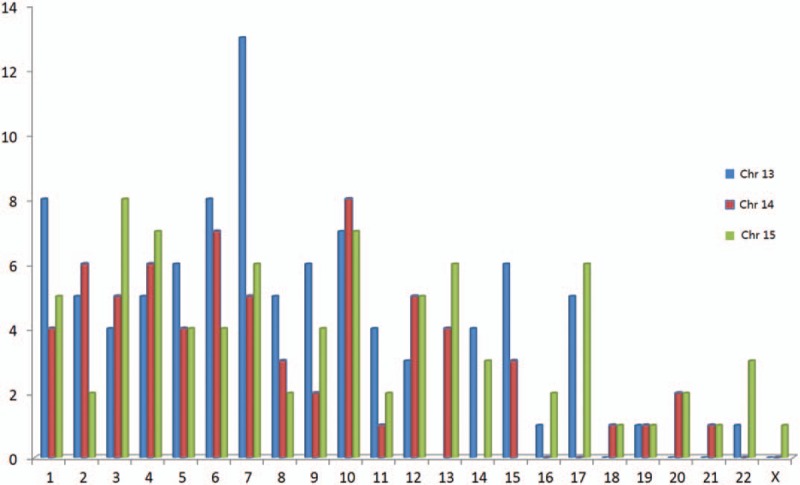
Distribution of chromosomes involved in translocations with chromosome 13, 14, and 15. Chr = chromosome.
The literature search identified 75 breakpoints. The most common breakpoint, at 13q22, was observed in 12 subjects, followed by 14q32 (n = 11), 15q15 (n = 9), and 15q22 (n = 9). Most breakpoints are related to gestational infertility, while breakpoints at 13p13, 13p12, 13p11.2, 13p11, 13q11, 13q15, 14p12, 14p10, 15p13, 15p10, and 15q22.2 are associated with pregestational infertility (see supplementary files: Table 2).
4. Discussion
Chromosomal abnormality is a major genetic factor contributing to male infertility.[2] Previous studies have reported that the presence of chromosomal translocations can alter the process of spermatogenesis.[17] Indeed, reciprocal and Robertsonian translocations have been shown to lead to male infertility or spontaneous abortion by altered segregation pattern, increased sperm aneuploidy, or altered semen parameters.[4,6,7,18] These effects are associated with specific chromosomes and breakpoints involved in translocation.[9] Balanced reciprocal translocation involving chromosomes 13, 14, or 15 are reported to be closely related to male infertility and recurrent pregnancy loss.[10,19,20] As male infertility is divided into pregestational and gestational infertility,[21] we divided the 28 subjects identified as carriers of balanced reciprocal translocation involving chromosomes 13, 14, or 15, and found 19 of these suffered pregestational infertility, the remaining 9 patients gestational infertility.
We similarly analyzed the literature and identified of the 201 subjects, 83 involving chromosomes 13, 56 involving chromosome 14, and 62 subjects involving chromosome 15. The most common translocations reported are t(7;13), t(10;14), and t(3;15), observed, respectively, in 13, 8, and 8 subjects. The non-Robertsonian translocations involving chromosomes 13, 14, or 15 are at increased risk of infertility or spontaneous abortions. Previous research has shown that abnormal synapsis in translocation carriers could lead to meiotic arrest and influence the spermatogenesis[19] by associated abnormal chromosome behavior with apoptosis in primary spermatocytes.[11]
A breakpoint in autosomal translocation may disrupt the genes responsible for spermatogenesis or impair the pairing of synaptic complexes during meiosis, thus resulting in reproductive failure.[22] To investigate the relationship between breakpoints in chromosomes 13, 14, and 15 and male infertility, we carried out an analysis of the related literature and identified a close association between breakpoints in these translocation carriers and male infertility and reproductive failure. In total, 75 breakpoints were identified. Of these, the most common breakpoint, at 13q22, was observed in 12 subjects, followed by 14q32 (n = 11), 15q15 (n = 9) and 15q22 (n = 9). Most breakpoints are related to gestational infertility, while breakpoints at 13p13, 13p12, 13p11.2, 13p11, 13q11, 13q15, 14p12, 14p10, 15p13, 15p10, and 15q22.2 are associated with pregestational infertility. Consequently, we recommend that patients undergoing genetic counseling for balanced translocation carriers should also receive preimplantation genetic diagnosis or prenatal testing.[23] In particular, the carriers of non-Robertsonian translocations involving chromosome 13, 14, or 15. A limitation of this study is the lack of detailed research regarding the specific molecular effects of each translocation by molecular-cytogenetic methods. Therefore, we are unable to explain the relationship between each breakpoint and spermatogenesis.
5. Conclusion
Our results show that 28 subjects are identified as carriers of balanced reciprocal translocation involving chromosomes 13, 14, or 15. Nineteen of these have experienced pregestational infertility, while 9 present with gestational infertility. Combined with literature analyses a total of 75 breakpoints are identified. Pregestational infertility is associated more of the chromosome 13 with the breakpoints at 13q14, while gestational infertility with 14q32. These differences have consequences for infertility treatment and genetic counseling. Intracytoplasmic sperm injection with PGD for the carriers with oligozoospermia, microscopic testicular sperm extraction or sperm from the sperm bank for the carriers with azoospermia should be considered for pregestational infertility. The carriers with gestational infertility can choose PGD or prenatal diagnosis.
Author contributions
HZ, 1st author, case analysis, and writing the article; RW,YY, clinical cases collection and analysis; HZ, 4th author, LL, cytogenetic analysis; XH, literature search; XY, data curation; RL, critical revision of the article; and final approval of article.
Data curation: Xiao Yang.
Funding acquisition: Ruizhi Liu.
Investigation: Ruixue Wang, Yang Yu.
Methodology: Haibo Zhu, Leilei Li.
Software: Xiaonan Hu.
Writing – original draft: Hongguo Zhang.
Writing – review & editing: Ruizhi Liu.
Supplementary Material
Footnotes
Abbreviations: CATSPER2 = Cation channel, sperm-associated, 2, KATNAL1 = Katanin, p60 subunit,a-like 1, PGD = preimplantation genetic diagnosis, PPIP5K1 = diphosphoinositol pentakisphosphate kinase 1, SPATA8 = spermatogenesis-associated protein 8, STRC = Stereocilin, TUBA3C = tubulin alpha 3c.
This work was supported by the National Natural Science Fund of China (81471515).
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
Nothing to disclose.
References
- [1].Dul EC, Groen H, van Ravenswaaij-Arts CM, et al. The prevalence of chromosomal abnormalities in subgroups of infertile men. Hum Reprod 2012;27:36–43. [DOI] [PubMed] [Google Scholar]
- [2].Gao M, Pang H, Zhao YH, et al. Karyotype analysis in large sample cases from Shenyang Women's and Children's hospital: a study of 16,294 male infertility patients. Andrologia 2017;49: [DOI] [PubMed] [Google Scholar]
- [3].Hotaling J, Carrell DT. Clinical genetic testing for male factor infertility: current applications and future directions. Andrology 2014;2:339–50. [DOI] [PubMed] [Google Scholar]
- [4].Pastuszek E, Kiewisz J, Kulwikowska PM, et al. Sperm parameters and DNA fragmentation of balanced chromosomal rearrangements carriers. Folia Histochem Cytobiol 2015;53:314–21. [DOI] [PubMed] [Google Scholar]
- [5].Suganya J, Kujur SB, Selvaraj K, et al. Chromosomal abnormalities in infertile men from southern India. J Clin Diagn Res 2015;9:GC05–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Godo A, Blanco J, Vidal F, et al. Altered segregation pattern and numerical chromosome abnormalities interrelate in spermatozoa from Robertsonian translocation carriers. Reprod Biomed Online 2015;31:79–88. [DOI] [PubMed] [Google Scholar]
- [7].Sobotka V, Vozdova M, Heracek J, et al. A rare Robertsonian translocation rob (14;22) carrier with azoospermia, meiotic defects, and testicular sperm aneuploidy. Syst Biol Reprod Med 2015;61:245–50. [DOI] [PubMed] [Google Scholar]
- [8].Harton GL, Tempest HG. Chromosomal disorders and male infertility. Asian J Androl 2012;14:32–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Godo A, Blanco J, Vidal F, et al. Accumulation of numerical and structural chromosome imbalances in spermatozoa from reciprocal translocation carriers. Hum Reprod 2013;28:840–9. [DOI] [PubMed] [Google Scholar]
- [10].Mikelsaar R, Nelis M, Kurg A, et al. Balanced reciprocal translocation t (5;13)(q33;q12) and 9q31.1 microduplication in a man suffering from infertility and pollinosis. J Appl Genet 2012;53:93–7. [DOI] [PubMed] [Google Scholar]
- [11].Jiang H, Wang L, Cui Y, et al. Meiotic chromosome behavior in a human male t(8;15) carrier. J Genet Genomics 2014;41:177–85. [DOI] [PubMed] [Google Scholar]
- [12].Smith LB, Milne L, Nelson N, et al. KATNAL1 regulation of sertoli cell microtubule dynamics is essential for spermiogenesis and male fertility. PLoS Genet 2012;8:e1002697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Jaiswal D, Singh V, Dwivedi US, et al. Chromosome microarray analysis: a case report of infertile brothers with CATSPER gene deletion. Gene 2014;542:263–5. [DOI] [PubMed] [Google Scholar]
- [14].Nie D, Xiang Y. Molecular cloning and characterization of a novel human testis-specific gene by use of digital differential display. J Genet 2006;85:57–62. [DOI] [PubMed] [Google Scholar]
- [15].Zhang ZH, Zhu HB, Li LL, et al. Decline of semen quality and increase of leukocytes with cigarette smoking in infertile men. Iran J Reprod Med 2013;11:589–96. [PMC free article] [PubMed] [Google Scholar]
- [16].Zhang HG, Wang RX, Li LL, et al. Male carriers of balanced reciprocal translocations in Northeast China: sperm count, reproductive performance, and genetic counseling. Genet Mol Res 2015;14:18792–8. [DOI] [PubMed] [Google Scholar]
- [17].Stouffs K, Seneca S, Lissens W. Genetic causes of male infertility. Ann Endocrinol (Paris) 2014;75:109–11. [DOI] [PubMed] [Google Scholar]
- [18].Zhao WW, Wu M, Chen F, et al. Robertsonian translocations: an overview of 872 Robertsonian translocations identified in a diagnostic laboratory in China. PLoS One 2015;10:e0122647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Ferguson KA, Chow V, Ma S. Silencing of unpaired meiotic chromosomes and altered recombination patterns in an azoospermic carrier of a t (8;13) reciprocal translocation. Hum Reprod 2008;23:988–95. [DOI] [PubMed] [Google Scholar]
- [20].Baccetti B, Bruni E, Collodel G, et al. 10, 15 reciprocal translocation in an infertile man: ultrastructural and fluorescence in-situ hybridization sperm study: case report. Hum Reprod 2003;18:2302–8. [DOI] [PubMed] [Google Scholar]
- [21].Li D, Zhang H, Wang R, et al. Chromosomal abnormalities in men with pregestational and gestational infertility in northeast China. J Assist Reprod Genet 2012;29:829–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Ananthapur V, Avvari S, Veena K, et al. Non-Robertsonian translocation t (2;11) is associated with infertility in an oligospermic man. Andrologia 2014;46:453–5. [DOI] [PubMed] [Google Scholar]
- [23].Vozdova M, Oracova E, Kasikova K, et al. Balanced chromosomal translocations in men: relationships among semen parameters, chromatin integrity, sperm meiotic segregation and aneuploidy. J Assist Reprod Genet 2013;30:391–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


