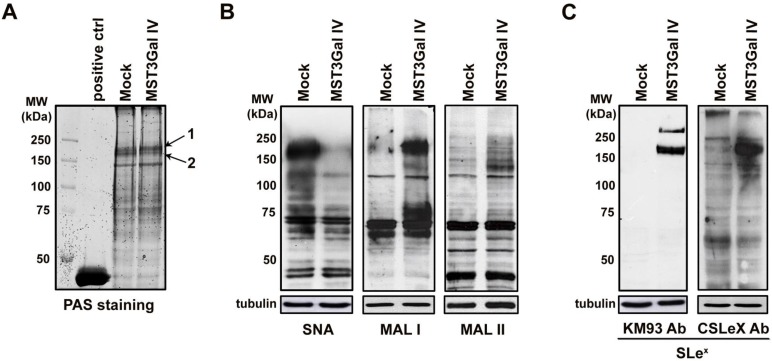
Figure 1.
Switching of protein sialylation in ST3Gal IV expressing gastric carcinoma cells leading to SLex expression. A - Periodic Acid-Schiff (PAS) staining of SDS-PAGE glycoproteins from Mock and MST3Gal IV total cell lysates showed no differences in total cell protein glycosylation on both cell lines; Horseradish peroxidase was used as positive staining control; Arrows 1 and 2 indicate the bands selected for protein identification by MALDI-TOF/TOF mass spectrometry. B - Evaluation of α2-6 and α2-3 linked NeuAc by SNA, MAL I and MAL II lectin staining on Mock and MST3Gal IV cells. Results showed a reduce intensity of α2-6 linked NeuAc carrying glycans accompanied by an increase in α2-3 linked NeuAc at high molecular weight protein in ST3Gal IV expressing cells. Mackia ammorensis lectins, capable of recognizing different α2-3 linked NeuAc carrying glycans structures, further demonstrated the presence of α2-3 linked NeuAc in Galβ1-4GlcNAc structures; C - Western blot analysis of SLeX structures using two different antibodies showed the presence of SLex antigens on high molecular weight proteins from ST3Gal IV expressing cells with no expression on Mock control cells.