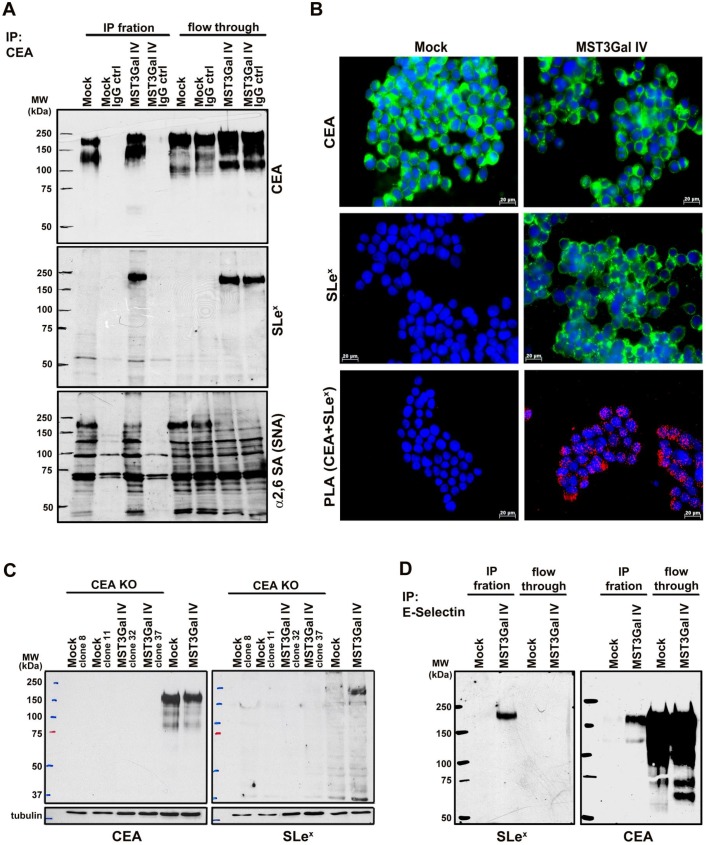
Figure 2.
CEA is the carrier of SLex in MST3Gal IV cell line. Four independent assays were employed to assess the presence SLex on CEA in Mock and MST3Gal IV cells: immunoprecipitation assay, PLA, CEA KO by CRISPR/Cas9 and E-selectin immunoprecipitation. A - CEA immunoprecipitation in MST3Gal IV and Mock cell lysates. Upper panel: CEA western blot analysis confirmed the expression of CEA in Mock and MST3Gal IV cells; middle panel: in MST3Gal IV cells the SLex epitope is largely attached to CEA glycans; lower panel: CEA expressed in MST3Gal IV cells showed a reduced presence of α2-6 NeuAc on CEA high molecular weight glycoforms. Immunoprecipitation flow through and a mouse IgG immunoprecipitation assay were used as experiment control. B - Immunofluorescence analysis confirmed the presence of CEA in Mock and MST3Gal IV gastric carcinoma cells while SLex is just found in the MST3Gal IV overexpressing cell line. The positive PLA signal only observed in MST3Gal IV cells is indicative of the close proximity of CEA and SLex. C - Knockout of CEA in both cell lines using CRISPR/Cas9 lead to the loss of SLex expression in MST3Gal IV cells. D - Immunoprecipitation of SLex carrying glycoproteins using E-selectin Fc protein chimera showed the high specificity of E-selectin for capturing SLex expressing glycoproteins in MST3Gal IV cells. A CEA western blot analysis after E-Selectin immunoaffinity enrichment was only positive for the MST3Gal IV cell lysates, clearly emphasizing the presence of SLeX on CEA.