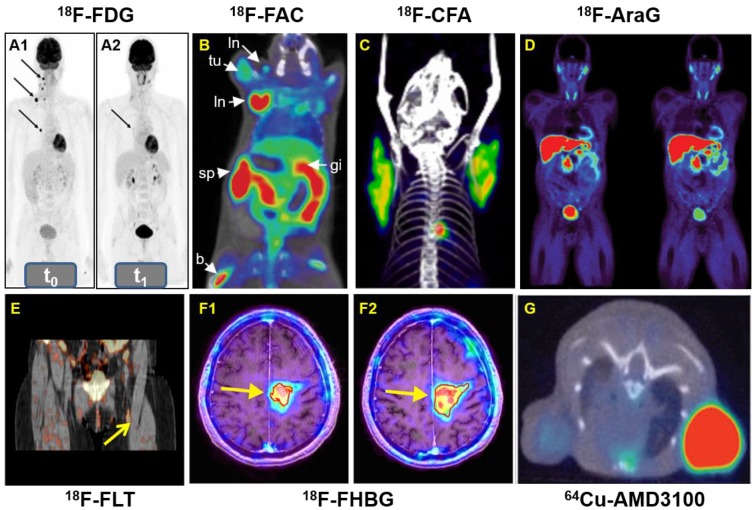
Figure 10.
A: Whole-body 18F-FDG-PET examination at two time points: before PD-1-therapy start (t0, base-line) (A1) and two weeks (t1, study examination) (A2). B: 18F-FAC was used to evaluate lymphoid organs and immune activation, tu: tumor; ln: lymph node; sp: spleen; gi: gastrointestinal tract; b: bone. C: Detection of immune responses after immunotherapy in glioblastoma using 18F-CFA. D: Pharmacokinetics of 18F-AraG in a healthy human volunteer to prepare to visualize activated T cells in acute graft-versus-host disease. E: Early identification of antigen-specific immune responses in vivo by18F-FLT. F: Reporter gene imaging of targeted T-cell immunotherapy in recurrent glioma by 18F-FHBG, F1: Pre-immunotherapy using CD8+ cytotoxic T lymphocytes (CTLs), F2: Post-immunotherapy using CD8+ CTLs. G: 64Cu-AMD3100-PET/CT for imaging of CXCR4 expression in subcutaneous brain tumor xenografts.