Fig. 2.
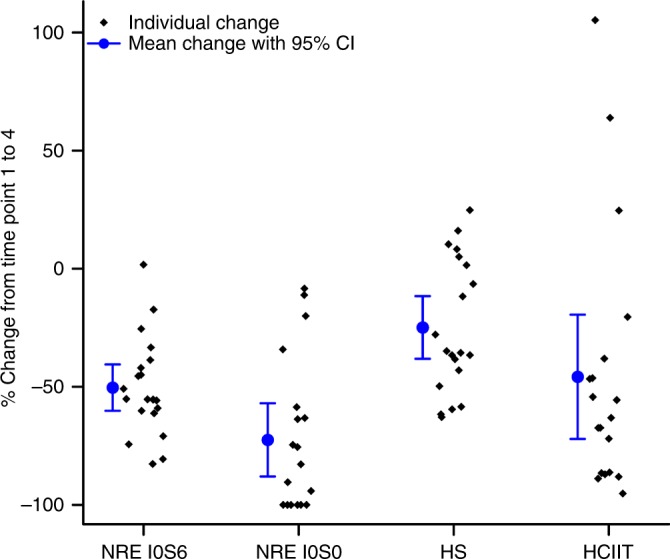
Decrease in biochemical abnormality from pretreatment to last follow-up. Percent change in biochemical abnormalities in the cerebrospinal fluid from time point 1 to 4, i.e., before any treatment to final intrathecal recombinant iduronidase infusion, 6 months posttransplant. All reductions were significant at p < 0.0001. Nonreducing ends (NREs) I0S0 and I0S6 (N = 20, N = 19, respectively) separately showed significant reductions, by more than 80% for I0S0 and 54% for I0S6. Heparan sulfate (HS, N = 20) decreased by 30%, and heparin cofactor II–thrombin complex (HCIIT, N = 19) decreased by more than half. CI confidence interval.
