Table 1.
Reaction optimization
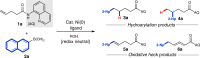
| |||||
|---|---|---|---|---|---|
| Entry | Ligand | Yield[%]a (3a:4a:5a:6a) | Entry | Ligand | Yield[%]a (3a:4a:5a:6a) |
| 1 | PPh3 | 47 (0/17/25/5) | 9 | PPhCy2 | 43 (0/10/29/4) |
| 2 | PCy3 | 41 (0/0/35/6) | 10 | P(OMe)3 | 30 (0/14/16/0) |
| 3 | PnBu3 | 39 (0/9/30/0) | 11 | PnPr3 | 29 (0/13/16/0) |
| 4 | PtBu3 | 25 (25/0/0/0) | 12 | PMe2Ph | 32 (0/25/7/0) |
| 5 | XPhos | 35 (35/0/0/0) | 13 | PtBuCy2 | 32 (0/15/17/0) |
| 6 | PMe3 | 85 (0/85/0/0) | 14 | Xantphos | 38 (0/16/19/3) |
| 7 | PPh2Cy | 44 (0/17/22/5) | 15b | PCy3 | 79 (0/0/79/0) |
| 8 | DCYPE | 0 | 16b | PnBu3 | 92 (0/0/92/0) |
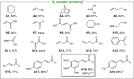
| |||||
Reactions conditions: Ni(cod)2 (10 mol%), ligand (20 mol%), CsOPiv (1.5 equiv), 1a (0.2 mmol), 2a (0.4 mmol), t-AmylOH (1 mL), 70 °C, 48 h
a1H NMR yields with C2H2Br4 as internal standard
bMolar ratio of 1a:2a = 1.5:1
cPnBu3 (20 mol%), H2 scavenger (2.0 equiv). The 1H NMR yield of 5a is given
d1.0 equiv H2 acceptor
eThe isolated yield is given in parenthesis
