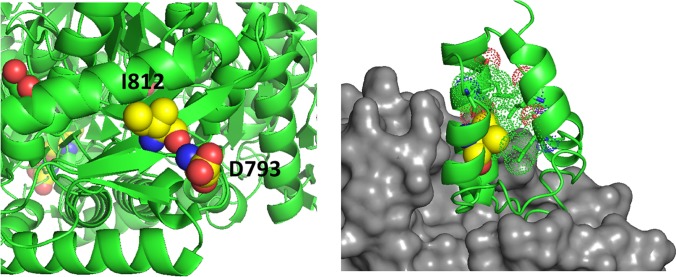
Figure 5.
Left panel, D793 and I812 are adjacent on parallel β-strands making backbone hydrogen bonds. Right panel, the structure of a phosphopantetheinyl transferase (gray surface) in complex with an ACP (acyl carrier protein) domain (green ribbon) shows that initial modification of the ACP domain serine (spheres) requires substantial access to the ACP surface. ACP helices 1 and 2 and the connecting loop lie on the surface of the transferase. The side chain of V330 (yellow spheres) packs in the interior of the ACP domain helical bundle. The substitution with Phe (rs2886059) will clash with surrounding residues (dots), likely causing a shift of the helix which contacts the transferase domain (gray surface) and interfering with binding.