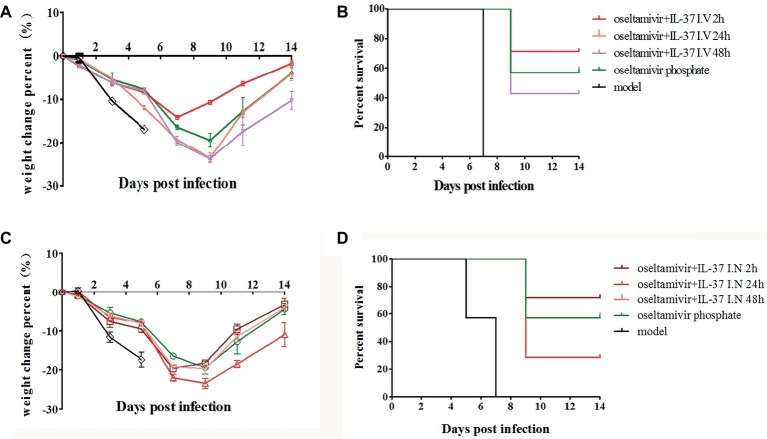
Figure 2.
IL-37 intravenous administration offers enhanced protection against influenza challenge in mice. BALB/c mice (n = 7 in each group) treated with or without IL-37 were divided into eight groups: the H1N1-infected group (model) and oseltamivir phosphate, intravenous oseltamivir phosphate combined with IL-37 at three separate time points (oseltamivir+IL-37 I. V 2 h, oseltamivir+IL-37 I. V 24 h, oseltamivir+IL-37 I. V 48 h) or intranasal oseltamivir phosphate combined with IL-37 at three separate time points (oseltamivir+IL-37 I. N 2 h, oseltamivir+IL-37 I. N 24 h, oseltamivir+IL-37 I. N 48 h) treatment groups. The body weights (A,C) and mortality rates (B,D) of mice treated via intravenous or intranasal routes were monitored. Data are presented as the average values from two independent experiments ± SD (n = 7 per group).